Prospective, randomised, parallel-group, open-label study to evaluate the effectiveness and safety of IMU-838, in combination with oseltamivir, in adults with COVID-19: the IONIC trial protocol
- PMID: 36396307
- PMCID: PMC9676417
- DOI: 10.1136/bmjopen-2021-055205
Prospective, randomised, parallel-group, open-label study to evaluate the effectiveness and safety of IMU-838, in combination with oseltamivir, in adults with COVID-19: the IONIC trial protocol
Abstract
Background: Globally, there is a scarcity of effective treatments for SARS-CoV-2 infections (causing COVID-19). Repurposing existing medications may offer the best hope for treating patients with COVID-19 to curb the pandemic. IMU-838 is a dihydroorotate dehydrogenase inhibitor, which is an effective mechanism for antiviral effects against respiratory viruses. When used synergistically with oseltamivir, therapeutic effects have been observed against influenza and SARS-CoV-2 in rodents. The IMU-838 and Oseltamivir in the Treatment of COVID-19 (IONIC) trial is a randomised controlled trial that will investigate whether time to clinical improvement in patients with COVID-19 is improved following a 14-day course of IMU-838+oseltamivir versus oseltamivir alone.
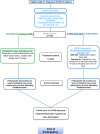
Methods: IONIC trial is an open-label study in which participants will be randomised 1:1 in two parallel arms: the intervention arm (IMU-838+oseltamivir) and the control arm (oseltamivir only). The primary outcome is time to clinical improvement; defined as the time from randomisation to a two-point improvement on WHO ordinal scale; discharge from hospital, or death (whichever occurs first). The study is sponsored by the University Hospitals Coventry and Warwickshire NHS Trust and funded by LifeArc.
Discussion: The IONIC protocol describes an overarching trial design to provide reliable evidence on the effectiveness of IMU-838 (vidofludimus calcium) when delivered in combination with an antiviral therapy (oseltamivir) (IONIC intervention) for confirmed or suspected COVID-19 infection in adult patients receiving usual standard of care.
Ethics and dissemination: This study has been independently reviewed and approved by Wales Research Ethics Committee. In addition, required regulatory approvals were received from Medicines and Healthcare products Regulatory Agency.
Trial registration number: EudraCT 2020-001805-21, ISRCTN53038326, NCT04516915.
Keywords: COVID-19; immunology; infectious diseases; public health.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous