Postnatal corticosteroid use for prevention or treatment of bronchopulmonary dysplasia in England and Wales 2012-2019: a retrospective population cohort study
- PMID: 36396314
- PMCID: PMC9676997
- DOI: 10.1136/bmjopen-2022-063835
Postnatal corticosteroid use for prevention or treatment of bronchopulmonary dysplasia in England and Wales 2012-2019: a retrospective population cohort study
Abstract
Objective: Describe the population of babies who do and do not receive postnatal corticosteroids for prevention or treatment of bronchopulmonary dysplasia (BPD).
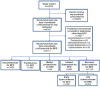
Design: Retrospective cohort study using data held in the National Neonatal Research Database.
Setting: National Health Service neonatal units in England and Wales.
Patients: Babies born less than 32 weeks gestation and admitted to neonatal units from 1 January 2012 to 31 December 2019.
Main outcomes: Proportion of babies given postnatal corticosteroid; type of corticosteroid; age at initiation and duration, trends over time.
Secondary outcomes: Survival to discharge, treatment for retinopathy of prematurity, BPD, brain injury, severe necrotising enterocolitis, gastrointestinal perforation.
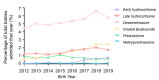
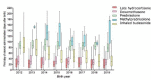
Results: 8% (4713/62019) of babies born <32 weeks and 26% (3525/13527) born <27 weeks received postnatal corticosteroids for BPD. Dexamethasone was predominantly used 5.3% (3309/62019), followed by late hydrocortisone 1.5%, inhaled budesonide 1.5%. prednisolone 0.8%, early hydrocortisone 0.3% and methylprednisolone 0.05%. Dexamethasone use increased over time (2012: 4.5 vs 2019: 5.8%, p=0.04). Median postnatal age of initiation of corticosteroid course was around 3 weeks for late hydrocortisone, 4 weeks for dexamethasone, 6 weeks for inhaled budesonide, 12 weeks for prednisolone and 16 weeks for methylprednisolone. Babies who received postnatal corticosteroids were born more prematurely, had a higher incidence of comorbidities and a longer length of stay.
Conclusions: In England and Wales, around 1 in 12 babies born less than 32 weeks and 1 in 4 born less than 27 weeks receive postnatal corticosteroids to prevent or treat BPD. Given the lack of convincing evidence of efficacy, challenges of recruiting to and length of time taken to conduct randomised controlled trial, our data highlight the need to monitor long-term outcomes in children who received neonatal postnatal corticosteroids.
Keywords: NEONATOLOGY; Neonatal intensive & critical care; RESPIRATORY MEDICINE (see Thoracic Medicine).
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: CG is funded by the UK Medical Research Council (MRC) through a Transition Support Award. He has received support from Chiesi Pharmaceuticals to attend an educational conference; in the past 5 years, he has been investigator on received research grants from Medical Research Council, National Institute of Health Research, Canadian Institute of Health Research, Department of Health in England, Mason Medical Research Foundation, Westminster Medical School Research Trust and Chiesi Pharmaceuticals. CG chairs the National Institute for Health and Care Research (NIHR) Research for Patient Benefit London Regional Panel. CB is funded by the UK NIHR through an Advanced Fellowship Award. She is deputy chair for the NIHR Prioritisation committee for Hospital-based care. CB has received support from Chiesi Pharmaceuticals to attend educational conferences.
Figures
Similar articles
-
Corticosteroids for the prevention and treatment of bronchopulmonary dysplasia: an overview of systematic reviews.Cochrane Database Syst Rev. 2024 Apr 10;4(4):CD013271. doi: 10.1002/14651858.CD013271.pub2. Cochrane Database Syst Rev. 2024. PMID: 38597338 Free PMC article. Review.
-
Optimising neonatal services for very preterm births between 27+0 and 31+6 weeks gestation in England: the OPTI-PREM mixed-methods study.Health Soc Care Deliv Res. 2025 Apr;13(12):1-126. doi: 10.3310/JYWC6538. Health Soc Care Deliv Res. 2025. PMID: 40232009
-
[The use of postnatal corticosteroid therapy in premature infants to prevent or treat bronchopulmonary dysplasia: current situation and recommendations].Arch Pediatr. 2010 Oct;17(10):1480-7. doi: 10.1016/j.arcped.2010.07.013. Epub 2010 Sep 22. Arch Pediatr. 2010. PMID: 20864322 French.
-
Influence of sex on the requirement for and outcomes following late postnatal corticosteroid treatment.Eur J Pediatr. 2023 Mar;182(3):1417-1423. doi: 10.1007/s00431-023-04826-3. Epub 2023 Jan 24. Eur J Pediatr. 2023. PMID: 36692623 Free PMC article.
-
Update on Postnatal Corticosteroids to Prevent or Treat Bronchopulmonary Dysplasia.Am J Perinatol. 2019 Jul;36(S 02):S58-S62. doi: 10.1055/s-0039-1691802. Epub 2019 Jun 25. Am J Perinatol. 2019. PMID: 31238361 Review.
Cited by
-
An updated review on systemic glucocorticoids in the prevention and treatment of bronchopulmonary dysplasia.Pediatr Discov. 2023 Jun 12;1(1):e6. doi: 10.1002/pdi3.6. eCollection 2023 Jun. Pediatr Discov. 2023. PMID: 40625572 Free PMC article. Review.
-
Glucocorticoid signature of preterm infants developing bronchopulmonary dysplasia.Pediatr Res. 2023 Nov;94(5):1804-1809. doi: 10.1038/s41390-023-02690-3. Epub 2023 Jun 24. Pediatr Res. 2023. PMID: 37355738
-
Systemic Postnatal Corticosteroids, Bronchopulmonary Dysplasia, and Survival Free of Cerebral Palsy.JAMA Pediatr. 2025 Jan 1;179(1):65-72. doi: 10.1001/jamapediatrics.2024.4575. JAMA Pediatr. 2025. PMID: 39556404
-
The impact of combined administration of surfactant and intratracheal budesonide compared to surfactant alone on bronchopulmonary dysplasia (BPD) and mortality rate in preterm infants with respiratory distress syndrome: a single-blind randomized clinical trial.BMC Pediatr. 2024 Apr 20;24(1):262. doi: 10.1186/s12887-024-04736-9. BMC Pediatr. 2024. PMID: 38643076 Free PMC article. Clinical Trial.
-
Temporal trends of the use of dexamethasone for the treatment of bronchopulmonary dysplasia in very low-birth-weight preterm infants: a single-center evaluation.Einstein (Sao Paulo). 2024 Nov 22;22:eAO0849. doi: 10.31744/einstein_journal/2024AO0849. eCollection 2024. Einstein (Sao Paulo). 2024. PMID: 39607113 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
