Evaluating the feasibility, effectiveness and costs of implementing person-centred follow-up care for childhood cancer survivors in four European countries: the PanCareFollowUp Care prospective cohort study protocol
- PMID: 36396317
- PMCID: PMC9677022
- DOI: 10.1136/bmjopen-2022-063134
Evaluating the feasibility, effectiveness and costs of implementing person-centred follow-up care for childhood cancer survivors in four European countries: the PanCareFollowUp Care prospective cohort study protocol
Abstract
Introduction: Long-term survival after childhood cancer often comes at the expense of late, adverse health conditions. However, survivorship care is frequently not available for adult survivors in Europe. The PanCareFollowUp Consortium therefore developed the PanCareFollowUp Care Intervention, an innovative person-centred survivorship care model based on experiences in the Netherlands. This paper describes the protocol of the prospective cohort study (Care Study) to evaluate the feasibility and the health economic, clinical and patient-reported outcomes of implementing PanCareFollowUp Care as usual care in four European countries.
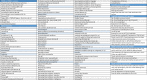
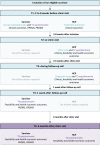
Methods and analysis: In this prospective, longitudinal cohort study with at least 6 months of follow-up, 800 childhood cancer survivors will receive the PanCareFollowUp Care Intervention across four study sites in Belgium, Czech Republic, Italy and Sweden, representing different healthcare systems. The PanCareFollowUp Care Intervention will be evaluated according to the Reach, Effectiveness, Adoption, Implementation and Maintenance framework. Clinical and research data are collected through questionnaires, a clinic visit for multiple medical assessments and a follow-up call. The primary outcome is empowerment, assessed with the Health Education Impact Questionnaire. A central data centre will perform quality checks, data cleaning and data validation, and provide support in data analysis. Multilevel models will be used for repeated outcome measures, with subgroup analysis, for example, by study site, attained age, sex or diagnosis.
Ethics and dissemination: This study will be conducted in accordance with the guidelines of Good Clinical Practice and the Declaration of Helsinki. The study protocol has been reviewed and approved by all relevant ethics committees. The evidence and insights gained by this study will be summarised in a Replication Manual, also including the tools required to implement the PanCareFollowUp Care Intervention in other countries. This Replication Manual will become freely available through PanCare and will be disseminated through policy and press releases.
Trial registration number: Netherlands Trial Register (NL8918; https://www.trialregister.nl/trial/8918).
Keywords: Health economics; International health services; Organisation of health services; Paediatric oncology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Vassal G, Schrappe M, Pritchard-Jones K, et al. . The SIOPE strategic plan: a European cancer plan for children and adolescents. J Cancer Policy 2016;8:17–32. 10.1016/j.jcpo.2016.03.007 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
