Treatment Effect of the SGLT2 Inhibitor Empagliflozin on Chronic Syndrome of Inappropriate Antidiuresis: Results of a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial
- PMID: 36396331
- PMCID: PMC10103093
- DOI: 10.1681/ASN.2022050623
Treatment Effect of the SGLT2 Inhibitor Empagliflozin on Chronic Syndrome of Inappropriate Antidiuresis: Results of a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial
Abstract
Background: The syndrome of inappropriate antidiuresis (SIAD) is characterized by a reduction of free water excretion with consecutive hypotonic hyponatremia and is therefore challenging to treat. The sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin promotes osmotic diuresis via urinary glucose excretion, likely leading to increased electrolyte free water clearance.
Methods: In this randomized, double-blind, placebo-controlled, crossover trial, we compared 4-week treatment with empagliflozin 25 mg/d to placebo in outpatients with chronic SIAD-induced hyponatremia. At baseline and after both treatment cycles, patients underwent different assessments including neurocognitive testing (Montreal Cognitive Assessment [MoCA]). The primary end point was the difference in serum sodium levels between treatments.
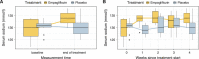
Results: Fourteen patients, 50% female, with a median age of 72 years (interquartile range [IQR], 65-77), completed the trial. Median serum sodium level at baseline was 131 mmol/L (IQR, 130-132). After treatment with empagliflozin, median serum sodium level rose to 134 mmol/L (IQR, 132-136), whereas no increase was seen with placebo (130 mmol/L; IQR, 128-132), corresponding to a serum sodium increase of 4.1 mmol/L (95% confidence interval [CI], 1.7 to 6.5; P =0.004). Exploratory analyses showed that treatment with empagliflozin led to improved neurocognitive function with an increase of 1.16 (95% CI, 0.05 to 2.26) in the MoCA score. Treatment was well tolerated; no serious adverse events were reported.
Conclusion: The SGLT2 inhibitor empagliflozin is a promising new treatment option for chronic SIAD-induced hyponatremia, possibly improving neurocognitive function. Larger studies are needed to confirm the observed treatment effects.
Clinical trial registration number: ClinicalTrials.gov NCT03202667.
Podcast: This article contains a podcast at.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
All authors have nothing to disclose.
Figures



Comment in
-
Food for thought: protein supplementation for the treatment of the syndrome of inappropriate antidiuresis.Eur J Endocrinol. 2023 Nov 8;189(5):R11-R14. doi: 10.1093/ejendo/lvad145. Eur J Endocrinol. 2023. PMID: 37930818 No abstract available.
References
-
- Bartter FC, Schwartz WB: The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med 42: 790–806, 1967 - PubMed
-
- Ellison DH, Berl T: Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 356: 2064–2072, 2007 - PubMed
-
- Verbalis JG, Greenberg A, Burst V, Haymann JP, Johannsson G, Peri A, et al. : Diagnosing and treating the syndrome of inappropriate antidiuretic hormone secretion. Am J Med 129: 537.e9–537.e23, 2016 - PubMed
-
- Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, et al. : Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med 126[Suppl 1]: S1–S42, 2013 - PubMed
-
- Spasovski G Vanholder R Allolio B Annane D Ball S Bichet D et al. ; Hyponatraemia Guideline Development Group : Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 170: G1–G47, 2014 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

