RS-FISH: precise, interactive, fast, and scalable FISH spot detection
- PMID: 36396787
- PMCID: PMC9718671
- DOI: 10.1038/s41592-022-01669-y
RS-FISH: precise, interactive, fast, and scalable FISH spot detection
Abstract
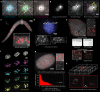
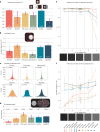
Fluorescent in-situ hybridization (FISH)-based methods extract spatially resolved genetic and epigenetic information from biological samples by detecting fluorescent spots in microscopy images, an often challenging task. We present Radial Symmetry-FISH (RS-FISH), an accurate, fast, and user-friendly software for spot detection in two- and three-dimensional images. RS-FISH offers interactive parameter tuning and readily scales to large datasets and image volumes of cleared or expanded samples using distributed processing on workstations, clusters, or the cloud. RS-FISH maintains high detection accuracy and low localization error across a wide range of signal-to-noise ratios, a key feature for single-molecule FISH, spatial transcriptomics, or spatial genomics applications.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

