Evaluation of cutaneous immune response in a controlled human in vivo model of mosquito bites
- PMID: 36396947
- PMCID: PMC9672097
- DOI: 10.1038/s41467-022-34534-9
Evaluation of cutaneous immune response in a controlled human in vivo model of mosquito bites
Abstract
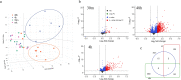
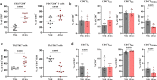
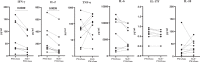
Mosquito-borne viruses are a growing global threat. Initial viral inoculation occurs in the skin via the mosquito 'bite', eliciting immune responses that shape the establishment of infection and pathogenesis. Here we assess the cutaneous innate and adaptive immune responses to controlled Aedes aegypti feedings in humans living in Aedes-endemic areas. In this single-arm, cross-sectional interventional study (trial registration #NCT04350905), we enroll 30 healthy adult participants aged 18 to 45 years of age from Cambodia between October 2020 and January 2021. We perform 3-mm skin biopsies at baseline as well as 30 min, 4 h, and 48 h after a controlled feeding by uninfected Aedes aegypti mosquitos. The primary endpoints are measurement of changes in early and late innate responses in bitten vs unbitten skin by gene expression profiling, immunophenotyping, and cytokine profiling. The results reveal induction of neutrophil degranulation and recruitment of skin-resident dendritic cells and M2 macrophages. As the immune reaction progresses T cell priming and regulatory pathways are upregulated along with a shift to Th2-driven responses and CD8+ T cell activation. Stimulation of participants' bitten skin cells with Aedes aegypti salivary gland extract results in reduced pro-inflammatory cytokine production. These results identify key immune genes, cell types, and pathways in the human response to mosquito bites and can be leveraged to inform and develop novel therapeutics and vector-targeted vaccine candidates to interfere with vector-mediated disease.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Specific human antibody responses to Aedes aegypti and Aedes polynesiensis saliva: A new epidemiological tool to assess human exposure to disease vectors in the Pacific.PLoS Negl Trop Dis. 2018 Jul 24;12(7):e0006660. doi: 10.1371/journal.pntd.0006660. eCollection 2018 Jul. PLoS Negl Trop Dis. 2018. PMID: 30040826 Free PMC article.
-
Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes.Immunology. 2019 Sep;158(1):47-59. doi: 10.1111/imm.13096. Immunology. 2019. PMID: 31315156 Free PMC article.
-
Antibodies to Aedes aegypti D7L salivary proteins as a new serological tool to estimate human exposure to Aedes mosquitoes.Front Immunol. 2024 May 1;15:1368066. doi: 10.3389/fimmu.2024.1368066. eCollection 2024. Front Immunol. 2024. PMID: 38751433 Free PMC article.
-
The biting rate of Aedes aegypti and its variability: A systematic review (1970-2022).PLoS Negl Trop Dis. 2023 Aug 8;17(8):e0010831. doi: 10.1371/journal.pntd.0010831. eCollection 2023 Aug. PLoS Negl Trop Dis. 2023. PMID: 37552669 Free PMC article.
-
Advancing the art of mosquito control: the journey of the sterile insect technique against Aedes aegypti in Cuba.Infect Dis Poverty. 2024 Aug 29;13(1):61. doi: 10.1186/s40249-024-01224-1. Infect Dis Poverty. 2024. PMID: 39198869 Free PMC article. Review.
Cited by
-
Multi-omics approaches for drug-response characterization in primary biliary cholangitis and autoimmune hepatitis variant syndrome.J Transl Med. 2024 Feb 29;22(1):214. doi: 10.1186/s12967-024-05029-6. J Transl Med. 2024. PMID: 38424613 Free PMC article.
-
T Cell Surveillance during Cutaneous Viral Infections.Viruses. 2024 Apr 26;16(5):679. doi: 10.3390/v16050679. Viruses. 2024. PMID: 38793562 Free PMC article. Review.
-
Structural and functional significance of Aedes aegypti AgBR1 flavivirus immunomodulator.J Virol. 2025 May 20;99(5):e0187824. doi: 10.1128/jvi.01878-24. Epub 2025 Apr 24. J Virol. 2025. PMID: 40272158 Free PMC article.
-
Aedes albopictus is not an arbovirus aficionado when feeding on cynomolgus macaques or squirrel monkeys.iScience. 2024 Oct 19;27(11):111198. doi: 10.1016/j.isci.2024.111198. eCollection 2024 Nov 15. iScience. 2024. PMID: 39555418 Free PMC article.
-
Aedes albopictus is not an arbovirus aficionado - Impacts of sylvatic flavivirus infection in vectors and hosts on mosquito engorgement on non-human primates.bioRxiv [Preprint]. 2024 Mar 11:2024.02.19.580944. doi: 10.1101/2024.02.19.580944. bioRxiv. 2024. PMID: 38559148 Free PMC article. Preprint.
References
-
- WHO. A Global Brief on Vector-Borne Diseases (WHO, 2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

