Protective potential of piroxicam on human peripheral blood mononuclear cells against the suppressive capacity of glioblastoma cell lines
- PMID: 36396965
- PMCID: PMC9672323
- DOI: 10.1038/s41598-022-24392-2
Protective potential of piroxicam on human peripheral blood mononuclear cells against the suppressive capacity of glioblastoma cell lines
Abstract
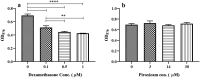
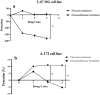
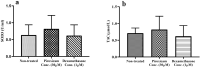
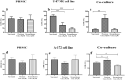
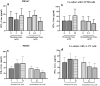
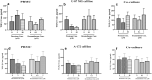
Dexamethasone, a common medication used in the treatment regimen of glioblastoma, has broad inhibitory effects on the immune responses. Here, in an in vitro study, we examined the effects of piroxicam, a potent substitute for dexamethasone, on peripheral blood mononuclear cells (PBMCs) co-cultured with two glioblastoma cell lines, U-87 MG and A-172 cells. MTT assay was used to determine the proliferation of PBMCs treated with piroxicam, or dexamethasone. In addition, to evaluate the effects of drugs on the cell cycle distribution, DNA content per cell was analyzed in PBMCs and A-172 cell lines using flow cytometry. Oxidative parameters, including superoxide dismutase-3 (SOD3) activity and total anti-antioxidant capacity, lactate dehydrogenase (LDH) activity, as well as IFN-γ and TGF-β levels were measured in PBMCs alone or in the presence of cell lines using ELISA. Unlike dexamethasone, piroxicam showed a protective effect on PBMCs against both glioblastoma cell lines. Furthermore, while dexamethasone reduced the proliferation of PBMCs, piroxicam had no adverse effect on the proliferation. Cell cycle analysis showed a reduction in the G2/M phase in piroxicam-treated A-172 cells. Additionally, dexamethasone limited the cell cycle progression by increasing the fraction of PBMCs in G0/G1. Interestingly, after co-culturing piroxicam-treated PBMCs with cell lines, a remarkable rise in the LDH activity was observed. Although not significant, piroxicam partially decreased TGF-β levels in both cell lines. Our findings suggested a protective effect of piroxicam, but not dexamethasone, on PBMCs against inhibitory mechanisms of two glioblastoma cell lines, U-87 and A-172 cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
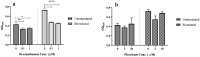


References
-
- Sasmita AO, Wong YP, Ling APK. Biomarkers and therapeutic advances in glioblastoma multiforme. Asia Pac. J. Clin. Oncol. 2018;14(1):40–51. - PubMed
-
- Stylli, S. S. Novel treatment strategies for glioblastoma. Multidisciplinary Digital Publishing Institute. p. 2883 (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

