Borrelia multiplex: a bead-based multiplex assay for the simultaneous detection of Borrelia specific IgG/IgM class antibodies
- PMID: 36396985
- PMCID: PMC9670078
- DOI: 10.1186/s12879-022-07863-9
Borrelia multiplex: a bead-based multiplex assay for the simultaneous detection of Borrelia specific IgG/IgM class antibodies
Abstract
Background: Lyme borreliosis (LB) is the most common tick-borne infectious disease in the northern hemisphere. The diagnosis of LB is usually made by clinical symptoms and subsequently supported by serology. In Europe, a two-step testing consisting of an enzyme-linked immunosorbent assay (ELISA) and an immunoblot is recommended. However, due to the low sensitivity of the currently available tests, antibody detection is sometimes inaccurate, especially in the early phase of infection, leading to underdiagnoses.
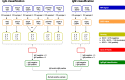
Methods: To improve upon Borrelia diagnostics, we developed a multiplex Borrelia immunoassay (Borrelia multiplex), which utilizes the new INTELLIFLEX platform, enabling the simultaneous dual detection of IgG and IgM antibodies, saving further time and reducing the biosample material requirement. In order to enable correct classification, the Borrelia multiplex contains eight antigens from the five human pathogenic Borrelia species known in Europe. Six antigens are known to mainly induce an IgG response and two antigens are predominant for an IgM response.
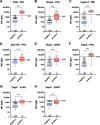
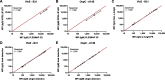
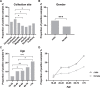
Results: To validate the assay, we compared the Borrelia multiplex to a commercial bead-based immunoassay resulting in an overall assay sensitivity of 93.7% (95% CI 84.8-97.5%) and a specificity of 96.5% (95%CI 93.5-98.1%). To confirm the calculated sensitivity and specificity, a comparison with a conventional 2-step diagnostics was performed. With this comparison, we obtained a sensitivity of 95.2% (95% CI 84.2-99.2%) and a specificity of 93.0% (95% CI 90.6-94.7%).
Conclusion: Borrelia multiplex is a highly reproducible cost- and time-effective assay that enables the profiling of antibodies against several individual antigens simultaneously.
Keywords: Borrelia; Immunoassay; Lyme Disease; Lyme borreliosis; Multiplex; Serology.
© 2022. The Author(s).
Conflict of interest statement
NSM was a speaker at Luminex conferences in the past. The Natural and Medical Sciences Institute at the University of Tübingen is involved in applied research projects as a fee for services with Luminex Corporation. The other authors declare no competing interests.
Figures

References
-
- Hunfeld K-P. Borrelien. In: Suerbaum S, Burchard G-D, Kaufmann SHE, Schulz TF, editors. Medizinische mikrobiologie und infektiologie. Berlin: Springer; 2020. pp. 487–498.
-
- Müllegger R. Infektionen: Lyme-Borreliose, Leptospirose und Rückfallfieber. In: Plewig G, Ruzicka T, Kaufmann R, Hertl M, editors. Braun-Falco’s dermatologie, venerologie und allergologie. Berlin: Springer; 2018. pp. 215–231.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

