Rotavirus vaccine clinical trials: a cross-sectional analysis of clinical trials registries
- PMID: 36397105
- PMCID: PMC9670083
- DOI: 10.1186/s13063-022-06878-6
Rotavirus vaccine clinical trials: a cross-sectional analysis of clinical trials registries
Abstract
Background: Rotavirus is a primary infectious virus causing childhood diarrhoea and is associated with significant mortality in children. Three African countries (Nigeria, the Democratic Republic of Congo, and Angola) are among the five countries that account for 50% of all diarrheal-related deaths worldwide. This indicates that much needs to be done to reduce this burden. The World Health Organization International Clinical Trial Registry Platform (WHO ICTRP) is a global repository for primary registries reporting on clinical trials. This study aimed to identify and describe planned, ongoing, and completed rotavirus vaccine trials conducted globally.
Methods: We searched WHO-ICTRP on 17 June 2021 and conducted a cross-sectional analysis of rotavirus studies listed in the database. Data extraction included trial location, participant age, source of the trial record, trial phase, sponsor, and availability of results. We used the Microsoft Excel 365 package to generate descriptive summary statistics.
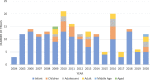
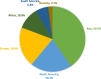
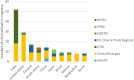
Results: We identified 242 rotavirus vaccine trials registered from 2004 to 2020. Most of these trials were registered retrospectively, with only 26% of the rotavirus vaccine trials reporting the availability of results in their registries. Most of the trials are studying children aged less than 5 years. The recruitment status for these trials is currently shown in the WHO-ICTRP as "not recruiting" for 80.17% of trials, "recruiting" for 11.57% of trials recruiting, and unknown for 6.61% of trials. The continents in which these rotavirus vaccine trials have recruitment sites in Asia (41%) and North America (20%), with the maximum number of trials in the clinical trial registries coming from India (21%) and the USA (11%) with most being sponsored by the pharmaceutical industry. Our analysis shows that only 26% of the rotavirus vaccine trials report the availability of results in their registries.
Conclusions: Mapping rotavirus vaccine clinical trial activity using data from the WHO ICTRP beneficial provides valuable information on planned, ongoing, or completed trials for researchers, funders, and healthcare decision-makers. Despite the high rotavirus disease burden in low- and middle-income countries, including Africa, there is minimal clinical trial activity related to the condition on the continent. The clinical trial registries as a valuable tool to share interim results of the trials.
Keywords: Clinical trial registry; Cross-sectionally analysis; Rotavirus vaccine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Human papillomavirus (HPV) trials: A cross-sectional analysis of clinical trials registries.Hum Vaccin Immunother. 2024 Dec 31;20(1):2393481. doi: 10.1080/21645515.2024.2393481. Epub 2024 Aug 28. Hum Vaccin Immunother. 2024. PMID: 39193782 Free PMC article.
-
Cholera vaccine clinical trials: A cross-sectional analysis of clinical trials registries.Hum Vaccin Immunother. 2023 Aug;19(2):2261168. doi: 10.1080/21645515.2023.2261168. Epub 2023 Sep 27. Hum Vaccin Immunother. 2023. PMID: 37759348 Free PMC article.
-
Human papillomavirus (HPV) vaccine clinical trials: A cross-sectional analysis of clinical trials registries.Hum Vaccin Immunother. 2025 Dec;21(1):2478702. doi: 10.1080/21645515.2025.2478702. Epub 2025 Mar 18. Hum Vaccin Immunother. 2025. PMID: 40101289
-
Rotavirus vaccines and vaccination in Latin America.Rev Panam Salud Publica. 2000 Nov;8(5):305-31. doi: 10.1590/s1020-49892000001000002. Rev Panam Salud Publica. 2000. PMID: 11190969 Review.
-
Update on Rotarix: an oral human rotavirus vaccine.Expert Rev Vaccines. 2009 Dec;8(12):1627-41. doi: 10.1586/erv.09.136. Expert Rev Vaccines. 2009. PMID: 19943758 Review.
Cited by
-
Development of RT-dPCR method and reference material for rotavirus G3P8 and G9P8.Anal Bioanal Chem. 2025 May;417(12):2513-2523. doi: 10.1007/s00216-024-05690-2. Epub 2024 Dec 16. Anal Bioanal Chem. 2025. PMID: 39676135
-
Immunogenicity and safety of a new hexavalent rotavirus vaccine in Chinese infants: A randomized, double-blind, placebo-controlled phase 2 clinical trial.Hum Vaccin Immunother. 2023 Aug;19(2):2263228. doi: 10.1080/21645515.2023.2263228. Epub 2023 Oct 16. Hum Vaccin Immunother. 2023. PMID: 37843437 Free PMC article. Clinical Trial.
-
Human papillomavirus (HPV) trials: A cross-sectional analysis of clinical trials registries.Hum Vaccin Immunother. 2024 Dec 31;20(1):2393481. doi: 10.1080/21645515.2024.2393481. Epub 2024 Aug 28. Hum Vaccin Immunother. 2024. PMID: 39193782 Free PMC article.
References
-
- Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JNM, Cheikh D, Fahmy K, Teleb N, Ashmony HA, Ahmed H, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health. 2019;7:e893–e903. doi: 10.1016/S2214-109X(19)30207-4. - DOI - PMC - PubMed
-
- Rotavirus vaccines WHO position paper: January 2013 - Recommendations. Vaccine. 2013;31(52):6170–1. 10.1016/j.vaccine.2013.05.037. Epub 2013 Jun 5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

