Effect of an enteral amino acid blend on muscle and gut functionality in critically ill patients: a proof-of-concept randomized controlled trial
- PMID: 36397118
- PMCID: PMC9670468
- DOI: 10.1186/s13054-022-04232-5
Effect of an enteral amino acid blend on muscle and gut functionality in critically ill patients: a proof-of-concept randomized controlled trial
Abstract
Background: A defining feature of prolonged critical illness is muscle wasting, leading to impaired recovery. Supplementation with a tailored blend of amino acids may bolster the innate gut defence, promote intestinal mucosa repair and limit muscle loss.
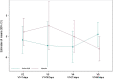
Methods: This was a monocentric, randomized, double-blind, placebo-controlled study that included patients with sepsis or acute respiratory distress syndrome. Patients received a specific combination of five amino acids or placebo mixed with enteral feeding for 21 days. Markers of renal function, gut barrier structure and functionality were collected at baseline and 1, 2, 3 and 8 weeks after randomization. Muscle structure and function were assessed through MRI measurements of the anterior quadriceps volume and by twitch airway pressure. Data were compared between groups relative to the baseline.
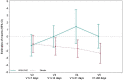
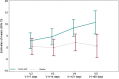
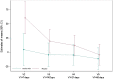
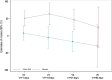
Results: Thirty-five critically ill patients were randomized. The amino acid blend did not impair urine output, blood creatinine levels or creatinine clearance. Plasma citrulline levels increased significantly along the treatment period in the amino acid group (difference in means [95% CI] 5.86 [1.72; 10.00] nmol/mL P = 0.007). Alanine aminotransferase and alkaline phosphatase concentrations were lower in the amino acid group than in the placebo group at one week (ratio of means 0.5 [0.29; 0.86] (P = 0.015) and 0.73 [0.57; 0.94] (P = 0.015), respectively). Twitch airway pressure and volume of the anterior quadriceps were greater in the amino acid group than in the placebo group 3 weeks after randomization (difference in means 10.6 [0.99; 20.20] cmH20 (P = 0.035) and 3.12 [0.5; 5.73] cm3/kg (P = 0.022), respectively).
Conclusions: Amino acid supplementation increased plasma citrulline levels, reduced alanine aminotransferase and alkaline phosphatase levels, and improved twitch airway pressure and anterior quadriceps volume. Trial registration ClinicalTrials.gov, NCT02968836. Registered November 21, 2016.
Keywords: Critical illness; Intensive care unit; Nutrition; Protein balance; Sepsis.
© 2022. The Author(s).
Conflict of interest statement
Nicholas Heming, Robert Carlier, Helene Prigent, Ahmed Mekki, Camille Jousset, Frederic Lofaso, Xavier Ambrosi, Rania Bounab, Virginie Maxime, Arnaud Mansart, Pascal Crenn, Pierre Moine and Djillali Annane declare no competing interests. Fabien Foltzer: former Nestlé employee, Bernard Cuenoud: Nestlé employee, Tobias Konz: former Nestlé employee, John Corthésy: Nestlé employee, Maurice Beaumont: former Nestlé employee, Claudia Roessle: former Nestlé employee, co-author of 2 patents filed by Nestlé Research in 2021 based on the results of the current study. Mickaël Hartweg: Nestlé employee, Jean-Charles Preiser: consultancy/speakers’ fees for Danone Nutricia/Fresenius/Nestlé HS. Denis Breuillé: Nestlé employee, co-author of 2 patents filed by Nestlé Research in 2021 based on the results of the current study.
Figures


References
-
- Reid C. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23:273–280. - PubMed
-
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. - PubMed
-
- Breuillé D, Arnal M, Rambourdin F, Bayle G, Levieux D, Obled C. Sustained modifications of protein metabolism in various tissues in a rat model of long-lasting sepsis. Clin Sci. 1998;94:413–423. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

