Adjuvant probiotic Bifidobacterium animalis subsp. lactis CP-9 improve phototherapeutic treatment outcomes in neonatal jaundice among full-term newborns: A randomized double-blind clinical study
- PMID: 36397441
- PMCID: PMC9666203
- DOI: 10.1097/MD.0000000000031030
Adjuvant probiotic Bifidobacterium animalis subsp. lactis CP-9 improve phototherapeutic treatment outcomes in neonatal jaundice among full-term newborns: A randomized double-blind clinical study
Abstract
Background: Probiotics had been used to decreased bilirubin level in neonatal jaundice (NJ) without being further studied mechanism and stratification. The intestinal pathogen Escherichia coli produced β-glucuronidase would increase enterohepatic circulation and elevate serum bilirubin levels (SBLs) which might worsen the disease process of NJ.
Study objective: We hypothesized that some probiotics could decrease bilirubin level through inhibiting the growth of E. coli. It's assumed that adjuvant probiotic intervention might accelerate the phototherapy for NJ and alleviate the severity of the NJ. Besides, it's further study the efficacy of the probiotic intervention in NJ among the full-term and preterm newborns.
Materials and methods: Firstly, the Bifidobacterium animalis subsp. lactis CP-9 was screened for its anti-E. coli activity. Then, it was orally administered to newborns with NJ in combination with conventional phototherapy (wavelength 425-457 nm) to determine its efficacy. 83 neonatal patients whose serum bilirubinemia was at a concentration of ≥ 15 mg/dL were participated the double-blind randomized trial and conducted in the neonatal ward of China Medical University Children's Hospital (CMUCH, Taichung, Taiwan). The test was conducted in 2 groups: experimental group: phototherapy + B. animalis subsp. lactis CP-9 (n = 43; 5 × 109 CFU/capsule) and control group: phototherapy + placebo (n = 40). The SBL and total phototherapy duration were measured.
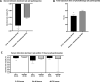
Results: The experimental group showed improved serum bilirubin decline rate (-0.16 ± 0.02 mg/dL/h; P = .009, 95% CI -0.12 to -0.2), particularly in the first 24 hour of in-hospital care, and reduced total phototherapy duration (44.82 ± 3.23 h; P = .011, 95% CI: 51.3-38.2) compared with the control group. Especially, probiotics had a significant therapeutic effect (serum bilirubin decline rate: -0.18 ± 0.02 mg/dL/h, 95% CI -0.12 to -0.23, P = .014; phototherapy duration: 43.17 ± 22.72 h, 95% CI 51.9-34.3, P = .019) in the low-risk subgroup (full-term newborns).
Conclusions: In conclusion, B. animalis subsp. lactis CP-9 synergistically improves treatment outcomes of NJ during in-hospital phototherapy including reduced total phototherapy duration and improved serum bilirubin decline rate, particularly in full-term newborns.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Figures



References
-
- Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med. 2017;78:699–704. - PubMed
-
- Gartner LM. Breastfeeding and jaundice. J Perinatol. 2001;21:25–9. - PubMed
-
- Chou SC, Palmer RH, Ezhuthachan S, et al. Management of hyperbilirubinemia in newborns: measuring performance by using a benchmarking model. Pediatrics. 2003;112:1264–73. - PubMed
-
- Tsao PC, Yeh HL, Chang YC, et al. Outcomes of neonatal jaundice in Taiwan. Arch Dis Child. 2018;103:927–9. - PubMed
-
- Wrong RJGH, Sibley DG. Therapy of unconjugated hyperbilirubinemia. 8 ed. Philadelphia: Mosby, 2006;1440–45.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

