Editing a gateway for cell therapy across the blood-brain barrier
- PMID: 36397727
- PMCID: PMC9976985
- DOI: 10.1093/brain/awac393
Editing a gateway for cell therapy across the blood-brain barrier
Abstract
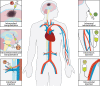
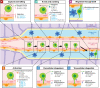
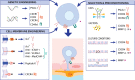
Stem cell therapy has been shown to improve stroke outcomes in animal models and is currently advancing towards clinical practice. However, uncertainty remains regarding the optimal route for cell delivery to the injured brain. Local intracerebral injections are effective in precisely delivering cells into the stroke cavity but carry the risk of damaging adjacent healthy tissue. Systemic endovascular injections, meanwhile, are minimally invasive, but most injected cells do not cross CNS barriers and become mechanically trapped in peripheral organs. Although the blood-brain barrier and the blood-CSF barrier tightly limit the entrance of cells and molecules into the brain parenchyma, immune cells can cross these barriers especially under pathological conditions, such as stroke. Deciphering the cell surface signature and the molecular mechanisms underlying this pathophysiological process holds promise for improving the targeted delivery of systemic injected cells to the injured brain. In this review, we describe experimental approaches that have already been developed in which (i) cells are either engineered to express cell surface proteins mimicking infiltrating immune cells; or (ii) cell grafts are preconditioned with hypoxia or incubated with pharmacological agents or cytokines. Modified cell grafts can be complemented with strategies to temporarily increase the permeability of the blood-brain barrier. Although these approaches could significantly enhance homing of stem cells into the injured brain, cell entrapment in off-target organs remains a non-negligible risk. Recent developments in safety-switch systems, which enable the precise elimination of transplanted cells on the administration of a drug, represent a promising strategy for selectively removing stem cells stuck in untargeted organs. In sum, the techniques described in this review hold great potential to substantially improve efficacy and safety of future cell therapies in stroke and may be relevant to other brain diseases.
Keywords: BBB; brain shuttle; iPSC; stem cells; stroke.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. - PubMed
-
- Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. - PubMed
-
- Weber F, Bohrmann B, Niewoehner J, et al. . Brain shuttle antibody for Alzheimer’s disease with attenuated peripheral effector function due to an inverted binding mode. Cell Rep. 2018;22:149–162. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

