A population-based study of factors associated with systemic treatment in advanced prostate cancer decedents
- PMID: 36397730
- PMCID: PMC10028120
- DOI: 10.1002/cam4.5401
A population-based study of factors associated with systemic treatment in advanced prostate cancer decedents
Abstract
Introduction: Life-prolonging therapies (LPTs) are rapidly evolving for the treatment of advanced prostate cancer, although factors associated with real-world uptake are not well characterized.
Methods: In this cohort of prostate-cancer decedents, we analyzed factors associated with LPT access. Population-level databases from Ontario, Canada identified patients 65 years or older with prostate cancer receiving androgen deprivation therapy and who died of prostate cancer between 2013 and 2017. Univariate and multivariable analyses assessed the association between baseline characteristics and receipt of LPT in the 2 years prior to death.
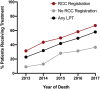
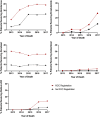
Results: Of 3575 patients who died of prostate cancer, 40.4% (n = 1443) received LPT, which comprised abiraterone (66.3%), docetaxel (50.3%), enzalutamide (17.2%), radium-223 (10.0%), and/or cabazitaxel (3.5%). Use of LPT increased by year of death (2013: 22.7%, 2014: 31.8%, 2015: 41.8%, 2016: 49.1%, and 2017: 57.9%, p < 0.0001), driven by uptake of all agents except docetaxel. Adjusted odds of use were higher for patients seen at Regional Cancer Centers (OR: 1.8, 95% CI: 1.5-2.1) and who received prior prostate-directed therapy (OR: 1.3, 95% CI: 1.0-1.5), but lower with advanced age (≥85: OR: 0.54, 95% CI:0.39-0.75), increased chronic conditions (≥6: OR: 0.62, 95% CI: 0.43-0.92), and long-term care residency (OR: 0.38, 95% CI: 0.17-0.89). Income, stage at presentation, and distance to the cancer center were not associated with LPT uptake.
Conclusion: In this cohort of prostate cancer-decedents, real-world uptake of novel prostate cancer therapies occurred at substantially higher rates for patients receiving care at Regional Cancer Centers, reinforcing the potential benefits for treatment access for patients referred to specialist centers.
Keywords: decedent; life-prolonging therapy; prostate cancer; regional cancer center.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
LTL is an advisory board participant for Sanofi, AbbVie, Janssen, Bayer, and Knight. DB has received an honorarium for advisory boards or speaker fees from Bayer, Janssen, Ipsen, Amgen, Pfizer, AstraZeneca, BMS, and AbbVie. MO has been a consultant to and received honoraria from Janssen and Bayer. All other authors have no conflicts to disclose.
Figures

References
-
- Ontario Cancer Statistics 2018 Report .” 2018. Cancer Care Ontario. January 8, 2018. https://www.cancercareontario.ca/en/statistical‐reports/ontario‐cancer‐s....
-
- Kirby M, Hirst C, Crawford ED. Characterising the castration‐resistant prostate cancer population: A systematic review. Int J Clin Pract. 2011;65(11):1180‐1192. - PubMed
-
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel‐T immunotherapy for castration‐resistant prostate cancer. New Eng J Med. 2010;363(5):411‐422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

