Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives
- PMID: 36397993
- PMCID: PMC9666035
- DOI: 10.1155/2022/3336516
Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives
Abstract
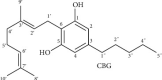
Cannabigerol (CBG) is a cannabinoid from the plant Cannabis sativa that lacks psychotomimetic effects. Its precursor is the acidic form, cannabigerolic acid (CBGA), which is, in turn, a biosynthetic precursor of the compounds cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC). CBGA decarboxylation leads to the formation of neutral cannabinoid CBG, through a chemical reaction catalyzed by heat. On the basis of the growing interest in CBG and with the aim of highlighting scientific information on this phytocannabinoid, we focused the content of this article on its pharmacokinetic and pharmacodynamic characteristics and on its principal pharmacological effects. CBG is metabolized in the liver by the enzyme CYP2J2 to produce hydroxyl and di-oxygenated products. CBG is considered a partial agonist at the CB1 receptor (R) and CB2R, as well as a regulator of endocannabinoid signaling. Potential pharmacological targets for CBG include transient receptor potential (TRP) channels, cyclooxygenase (COX-1 and COX-2) enzymes, cannabinoid, 5-HT1A, and alpha-2 receptors. Pre-clinical findings show that CBG reduces intraocular pressure, possesses antioxidant, anti-inflammatory, and anti-tumoral activities, and has anti-anxiety, neuroprotective, dermatological, and appetite-stimulating effects. Several findings suggest that research on CBG deserves to be deepened, as it could be used, alone or in association, for novel therapeutic approaches for several disorders.
Copyright © 2022 Fabrizio Calapai et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Gaoni Y., Mechoulan R. Structure synthesis of cannabigerol new hashish constituent. Proceedings of the Chemical Society, London . 1964;82:2189–2192.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous