Enteral gabexate mesilate improves volume requirements and autonomic cardiovascular function after experimental trauma/hemorrhagic shock in the absence of blood reperfusion
- PMID: 36398214
- PMCID: PMC9641450
Enteral gabexate mesilate improves volume requirements and autonomic cardiovascular function after experimental trauma/hemorrhagic shock in the absence of blood reperfusion
Abstract
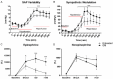
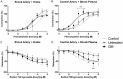
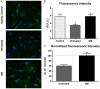
The standard of care for fluid resuscitation of trauma/hemorrhagic shock (T/HS) is the infusion of blood. However, in many instances, blood product transfusion may not be feasible. Consequently, crystalloid solutions may be utilized as temporizing cost-effective resuscitation fluids. In this study, we explored an alternative therapeutic strategy of enteral protease inhibition adjunctive to intravenous Lactated Ringer's (LR) reperfusion after T/HS. Male Wistar rats underwent midline laparotomy (trauma) and an enteral catheter was inserted orally and positioned post-pyloric for the infusion of vehicle (Golytely®) with or without the serine protease inhibitor gabexate mesilate (GM) (n=8/group). Hemorrhagic shock was induced by blood removal to reduce the mean arterial blood pressure (MAP) to 35-40 mmHg for 90 minutes, before resuscitation with LR. Animals treated with enteral GM required significantly less crystalloid volume to achieve hemodynamic stability and displayed improvements in both blood pressure and autonomic function (via increased baroreflex sensitivity to vasopressors, heightened vascular sympathetic modulation, elevated levels of circulating catecholamines, and increased α1-adrenergic receptor density) compared to untreated (control) shocked animals. Resistance arteries isolated from healthy donor animals and perfused with plasma from untreated T/HS animals revealed impaired vascular response to the α1 adrenergic agonist phenylephrine and decreased reactivity to sodium nitroprusside that was preserved in the GM-treated group. These findings suggest that blockade of serine proteases within the intestinal lumen in non-blood resuscitated experimental T/HS preserves and enhances peripheral sympathetic modulation, improving hemodynamics. Enteral infusion of gabexate mesilate may be a new and promising approach to the management of trauma/hemorrhagic shock.
Keywords: Trauma/hemorrhagic shock; autonomic cardiovascular function; enteral protease inhibition; gabexate mesilate.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures


References
-
- Stewart RM, Myers JG, Dent DL, Ermis P, Gray GA, Villarreal R, Blow O, Woods B, McFarland M, Garavaglia J, Root HD, Pruitt BA Jr. Seven hundred fifty-three consecutive deaths in a level I trauma center: the argument for injury prevention. J Trauma. 2003;54:66–70. discussion 70-1. - PubMed
-
- Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, Bolenbaucher R, Blackbourne LH. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71:S4–8. - PubMed
-
- Eastridge BJ, Holcomb JB, Shackelford S. Outcomes of traumatic hemorrhagic shock and the epidemiology of preventable death from injury. Transfusion. 2019;59:1423–1428. - PubMed
-
- Cooke WH, Salinas J, Convertino VA, Ludwig DA, Hinds D, Duke JH, Moore FA, Holcomb JB. Heart rate variability and its association with mortality in prehospital trauma patients. J Trauma. 2006;60:363–370. discussion 370. - PubMed
