Influence of glimepiride plus sitagliptin on treatment outcome, blood glucose, and oxidative stress in diabetic patients
- PMID: 36398218
- PMCID: PMC9641479
Influence of glimepiride plus sitagliptin on treatment outcome, blood glucose, and oxidative stress in diabetic patients
Abstract
Objective: This research sets out to investigate the influence of glimepiride (GLIM) plus sitagliptin (SITA) on the treatment outcome, blood glucose (BG), and oxidative stress (OS) in diabetic patients.
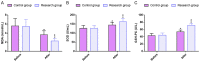
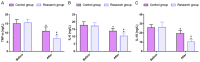
Methods: In this retrospective study, 189 patient cases of type 2 diabetes mellitus (T2DM) admitted from July 2017 to July 2021 to the Affiliated Hospital of Nantong University were selected, of whom 99 cases treated with GLIM + SITA were assigned to the research group (RG) and 90 cases receiving GLIM monotherapy were set as the control group (CG). The two cohorts of patients were compared in terms of treatment outcomes, BG, islet function, OS, inflammatory responses (IRs), and safety. The BG indexes detected mainly included fasting blood glucose (FBG), 2-hour postprandial blood glucose (2hPG) and glycosylated hemoglobin (HbA1c). Islet function was mainly measured by Homeostasis Model Assessment of β-cell Function (HOMA-β) and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR). The OS parameters measured primarily included malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX). Tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-18 were the inflammatory factors measured.
Results: A statistically higher excellent or good rate of treatment was determined in the RG compared to the CG. After treatment, FBG, 2hPG, HbA1c, HOMA-IR, MDA, TNF-α, IL-6, and IL-18 were lower in the RG while HOMA-β, SOD, and GSH-PX were higher, compared to the levels before treatment and the CG. A non-significantly different incidence of adverse reactions between groups was determined.
Conclusions: Our findings demonstrated high efficacy of GLIM + SITA in the treatment of T2DM patients, which can effectively improve the BG and OS of patients and reduce inflammation without increasing the incidence of adverse reactions. This should have high clinical application value.
Keywords: Glimepiride; blood glucose; diabetes; inflammatory response; oxidative stress; sitagliptin; treatment outcome.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures


Similar articles
-
Concurrent alteration in inflammatory biomarker gene expression and oxidative stress: how aerobic training and vitamin D improve T2DM.BMC Complement Med Ther. 2022 Jun 22;22(1):165. doi: 10.1186/s12906-022-03645-7. BMC Complement Med Ther. 2022. PMID: 35733163 Free PMC article. Clinical Trial.
-
[Effects of danzhi jiangtang Capsule combined exercise on pancreatic oxidative stress and islet beta-cell function in diabetic rats].Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012 Nov;32(11):1531-4. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012. PMID: 23359980 Chinese.
-
Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients.Metabolism. 2010 Jun;59(6):887-95. doi: 10.1016/j.metabol.2009.10.007. Epub 2009 Dec 16. Metabolism. 2010. PMID: 20015525 Clinical Trial.
-
Probiotics improve renal function, glucose, lipids, inflammation and oxidative stress in diabetic kidney disease: a systematic review and meta-analysis.Ren Fail. 2022 Dec;44(1):862-880. doi: 10.1080/0886022X.2022.2079522. Ren Fail. 2022. PMID: 35611435 Free PMC article.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
Cited by
-
Helical-Like Assembly of Nateglinide as Coating for Oral Delivery of Insulin and Their Synergistic Prevention of Diabetes Mellitus.Adv Sci (Weinh). 2023 Oct;10(29):e2301879. doi: 10.1002/advs.202301879. Epub 2023 Aug 16. Adv Sci (Weinh). 2023. PMID: 37587777 Free PMC article.
-
Effects of sitagliptin activation of the stromal cell-derived factor-1/CXC chemokine receptor 4 signaling pathway on the proliferation, apoptosis, inflammation, and osteogenic differentiation of human periodontal ligament stem cells induced by lipopolysaccharide.Hua Xi Kou Qiang Yi Xue Za Zhi. 2024 Feb 1;42(1):37-45. doi: 10.7518/hxkq.2024.2023213. Hua Xi Kou Qiang Yi Xue Za Zhi. 2024. PMID: 38475949 Free PMC article. Chinese, English.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
