Triamcinolone Acetonide Suprachoroidal Injectable Suspension for Uveitic Macular Edema: Integrated Analysis of Two Phase 3 Studies
- PMID: 36399237
- PMCID: PMC9834475
- DOI: 10.1007/s40123-022-00603-x
Triamcinolone Acetonide Suprachoroidal Injectable Suspension for Uveitic Macular Edema: Integrated Analysis of Two Phase 3 Studies
Abstract
Introduction: Macular edema, a common complication of uveitis, may result in vision loss. The aim of this analysis was to report integrated phase 3 trial data for triamcinolone acetonide injectable suspension for suprachoroidal use (SCS-TA) in the treatment of macular edema secondary to noninfectious uveitis using strict inclusion criteria.
Methods: This analysis included patients with central subfield thickness (CST) ≥ 300 µm and best-corrected visual acuity (BCVA) of ≥ 5 and ≤ 70 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at both screening and baseline who received ≥ 1 study treatment in either PEACHTREE (randomized, double-masked SCS-TA or sham control) or AZALEA (open-label SCS-TA). Patients received SCS-TA 4.0 mg (0.1 ml of 40 mg/ml) or control at baseline and week 12.
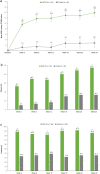
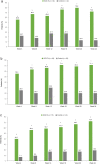
Results: In the SCS-TA group (n = 95), 47.4% of patients gained ≥ 15 ETDRS letters from baseline to week 24 versus 16.7% of patients in the control group (n = 60; P < 0.001). Mean change in BCVA in the SCS-TA group was 9.6 letters at week 4 and 13.9 letters at week 24. CST also improved rapidly in the SCS-TA group (mean change: - 158.4 µm at week 4), with sustained reduction throughout the study (mean change: - 163.9 µm at week 24 versus - 19.3 µm in the control group; P < 0.001). No treatment-related serious adverse events (AEs) were reported. Incidence of AEs pertaining to elevated intraocular pressure was 12.6% and 15.0% in the SCS-TA and control groups, respectively; incidence of cataract formation/worsening AEs was 7.4% and 6.7%, respectively.
Conclusion: In this integrated analysis utilizing strict inclusion criteria, SCS-TA was found effective in the treatment of patients with macular edema associated with noninfectious uveitis and was generally well tolerated.
Trial registration: ClinicalTrials.gov identifier: NCT02595398, NCT03097315.
Keywords: Central subfield thickness; Macular edema; Suprachoroidal; Triamcinolone acetonide; Uveitis.
© 2022. The Author(s).
Figures


References
-
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

