Development of a Novel HTLV-1 Protease: Human Fcγ1 Recombinant Fusion Molecule in the CHO Eukaryotic Expression System
- PMID: 36399306
- PMCID: PMC9673214
- DOI: 10.1007/s12010-022-04259-y
Development of a Novel HTLV-1 Protease: Human Fcγ1 Recombinant Fusion Molecule in the CHO Eukaryotic Expression System
Abstract
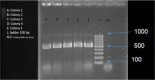
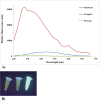
Human T-cell leukaemia virus type 1 (HTLV-1) is the causative agent of two life-threatening diseases, adult T cell leukaemia/lymphoma (ATLL), and HTLV-1-associated myelopathy/tropical spastic (HAM/TSP). HTLV-1 protease (HTLV-1-PR) is an aspartic protease that represents a promising target for therapeutic purposes like human immunodeficiency virus-PR inhibitors (HIV-PR). Therefore, in this study, the human Fc fusion recombinant-PR (HTLV-1-PR:hFcγ1) was designed and expressed for two applications, finding a blocking substrate as a potential therapeutic or a potential subunit peptide vaccine. The PCR amplified DNA sequences encoding the HTLV-1-PR from the MT2-cell line using specific primers with restriction enzyme sites of Not1 and Xba1. The construct was then cloned to pTZ57R/T TA plasmid and, after confirming the PR sequence, subcloned into the pDR2ΔEF1α Fc-expression vector to create pDR2ΔEF1α.HTLV-1-PR:hFcγ1. The integrity of recombinant DNA was confirmed by sequencing to ensure that the engineered construct was in the frame. The recombinant fusion protein was then produced in the Chinese hamster ovary cell (CHO) system and was purified from its supernatant using HiTrap-rPA column affinity chromatography. Then, the immunofluorescence assay (IFA) co-localisation method showed that HTLV-1-PR:hFc recombinant fusion protein has appropriate folding as it binds to the anti-Fcγ antibody; the Fcγ1 tag participates to have HTLV-1-PR:hFcγ1 as a dimeric secretory protein. The development and production of HTLV-1-PR can be used to find a blocking substrate as a potential therapeutic molecule and apply it in an animal model to assess its immunogenicity and potential protection against HTLV-1 infection.
Keywords: Adult T cell leukaemia/lymphoma (ATLL); Chinese hamster ovary cell (CHO) expression system; HTLV-1 protease; Human T-cell leukaemia virus type 1 (HTLV-1); Recombinant Fc-fusion protein.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Mycobacterium tuberculosis Ag85b:hfcγ1 recombinant fusion protein as a selective receptor-dependent delivery system for antigen presentation.Microb Pathog. 2019 Apr;129:68-73. doi: 10.1016/j.micpath.2019.01.045. Epub 2019 Jan 31. Microb Pathog. 2019. PMID: 30711546
-
Construction of Mycobacterium tuberculosis ESAT-6 fused to human Fcγ of IgG1: To target FcγR as a delivery system for enhancement of immunogenicity.Gene. 2016 Apr 15;580(2):111-117. doi: 10.1016/j.gene.2016.01.009. Epub 2016 Jan 8. Gene. 2016. PMID: 26778208
-
Selective APC-targeting of a novel Fc-fusion multi-immunodominant recombinant protein (tTax-tEnv:mFcγ2a) for HTLV-1 vaccine development.Life Sci. 2022 Nov 1;308:120920. doi: 10.1016/j.lfs.2022.120920. Epub 2022 Aug 28. Life Sci. 2022. PMID: 36044973
-
Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects.Rev Neurol (Paris). 2012 Mar;168(3):257-69. doi: 10.1016/j.neurol.2011.12.006. Epub 2012 Mar 7. Rev Neurol (Paris). 2012. PMID: 22405461 Review.
-
Human T-lymphotropic virus type 1 (HTLV-1) and cellular immune response in HTLV-1-associated myelopathy/tropical spastic paraparesis.J Neurovirol. 2020 Oct;26(5):652-663. doi: 10.1007/s13365-020-00881-w. Epub 2020 Jul 23. J Neurovirol. 2020. PMID: 32705480 Free PMC article. Review.
References
-
- Ghezeldasht SA, Shirdel A, Assarehzadegan MA, Hassannia T, Rahimi H, Miri R, Rezaee SR. Human T lymphotropic virus type I (HTLV-I) oncogenesis: Molecular aspects of virus and host interactions in pathogenesis of adult T cell leukemia/lymphoma (ATL) Iranian Journal of Basic Medical Sciences. 2013;16(3):179. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

