The management of congenital adrenal hyperplasia during preconception, pregnancy, and postpartum
- PMID: 36399318
- PMCID: PMC9884653
- DOI: 10.1007/s11154-022-09770-5
The management of congenital adrenal hyperplasia during preconception, pregnancy, and postpartum
Abstract
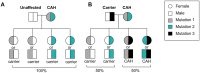
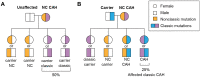
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders of steroidogenesis of the adrenal cortex, most commonly due to 21-hydroxylase deficiency caused by mutations in the CYP21A2 gene. Although women with CAH have decreased fecundity, they are able to conceive; thus, if pregnancy is not desired, contraception options should be offered. If fertility is desired, women with classic CAH should first optimize glucocorticoid treatment, followed by ovulation induction medications and gonadotropins if needed. Due to the possible pregnancy complications and implications on the offspring, preconception genetic testing and counseling with a high-risk obstetrics specialist is recommended. For couples trying to avoid having a child with CAH, care with a reproductive endocrinology and infertility specialist to utilize in vitro fertilization can be offered, with or without preimplantation genetic testing for monogenic disorders. Prenatal screening and diagnosis options during pregnancy include maternal serum cell free-DNA for sex of the baby, and chorionic villus sampling and amniocentesis for diagnosis of CAH. Pregnant women with classic CAH need glucocorticoids to be adjusted during the pregnancy, at the time of delivery, and postpartum, and should be monitored for adrenal crisis. Maternal and fetal risks may include chorioamnionitis, maternal hypertension, gestational diabetes, cesarean section, and small for gestational age infants. This review on CAH due to 21-hydroxylase deficiency highlights reproductive health including genetic transmission, contraception options, glucocorticoid management, fertility treatments, as well as testing, antenatal monitoring, and management during pregnancy, delivery, and postpartum.
Keywords: 21-hydroxylase deficiency; Congenital adrenal hyperplasia; Contraception; Fertility; Pregnancy.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
D.P.M. received research funds from Diurnal Limited through the National Institutes of Health Cooperative Research and Development Agreement. J.Y.M. and V.G.L. have nothing to disclose.
Figures


References
-
- Merke DP, Auchus RJ. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. N Engl J Med. 2020;383(13):1248–1261. - PubMed
-
- El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194–2210. - PubMed
-
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(11):4043–4088. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

