Disproportionation and Ligand Lability in Low Oxidation State Boryl-Tin Chemistry
- PMID: 36399407
- PMCID: PMC10947314
- DOI: 10.1002/chem.202203395
Disproportionation and Ligand Lability in Low Oxidation State Boryl-Tin Chemistry
Abstract
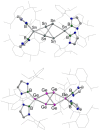
Boryltin compounds featuring the metal in the+1 or 0 oxidation states can be synthesized from the carbene-stabilized tin(II) bromide (boryl)Sn(NHC)Br (boryl={B(NDippCH)2 }; NHC=C{(Ni PrCMe)2 }) by the use of strong reducing agents. The formation of the mono-carbene stabilized distannyne and donor-free distannide systems (boryl)SnSn(IPrMe)(boryl) (2) and K2 [Sn2 (boryl)2 ] (3), using Mg(I) and K reducing agents mirrors related germanium chemistry. In contrast to their lighter congeners, however, systems of the type [Sn(boryl)]n are unstable with respect to disproportionation. Carbene abstraction from 2 using BPh3 , and two-electron oxidation of 3 both result in the formation of a 2 : 1 mixture of the Sn(II) compound Sn(boryl)2 , and the hexatin cluster, Sn6 (boryl)4 (4). A viable mechanism for this rearrangement is shown by quantum chemical studies to involve a vinylidene intermediate (analogous to the isolable germanium compound, (boryl)2 Ge=Ge), which undergoes facile atom transfer to generate Sn(boryl)2 and trinuclear [Sn3 (boryl)2 ]. The latter then dimerizes to give the observed hexametallic product 4, with independent studies showing that similar trigermanium species aggregate in analogous fashion.
Keywords: boryl ligand; cluster; germanium; sub-valent compounds; tin.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Phillips A. D., Wright R. J., Olmstead M. M., Power P. P., J. Am. Chem. Soc. 2002, 124, 5930–5931. - PubMed
-
- Stender M., Phillips A. D., Wright R. J., Power P. P., Angew. Chem. Int. Ed. 2002, 41, 1785–1787. - PubMed
-
- Power P. P., Nature 2010, 463, 171–177. - PubMed
-
- None
-
- Lansing E., Kenneth Pitzer B. S., The Thermodynamic and Physical Properties of the Elements, Cornell University Press, 1947;
Grants and funding
LinkOut - more resources
Full Text Sources

