Dissecting the invasion of Galleria mellonella by Yersinia enterocolitica reveals metabolic adaptations and a role of a phage lysis cassette in insect killing
- PMID: 36399504
- PMCID: PMC9718411
- DOI: 10.1371/journal.ppat.1010991
Dissecting the invasion of Galleria mellonella by Yersinia enterocolitica reveals metabolic adaptations and a role of a phage lysis cassette in insect killing
Abstract
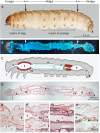
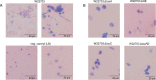
The human pathogen Yersinia enterocolitica strain W22703 is characterized by its toxicity towards invertebrates that requires the insecticidal toxin complex (Tc) proteins encoded by the pathogenicity island Tc-PAIYe. Molecular and pathophysiological details of insect larvae infection and killing by this pathogen, however, have not been dissected. Here, we applied oral infection of Galleria mellonella (Greater wax moth) larvae to study the colonisation, proliferation, tissue invasion, and killing activity of W22703. We demonstrated that this strain is strongly toxic towards the larvae, in which they proliferate by more than three orders of magnitude within six days post infection. Deletion mutants of the genes tcaA and tccC were atoxic for the insect. W22703 ΔtccC, in contrast to W22703 ΔtcaA, initially proliferated before being eliminated from the host, thus confirming TcaA as membrane-binding Tc subunit and TccC as cell toxin. Time course experiments revealed a Tc-dependent infection process starting with midgut colonisation that is followed by invasion of the hemolymph where the pathogen elicits morphological changes of hemocytes and strongly proliferates. The in vivo transcriptome of strain W22703 shows that the pathogen undergoes a drastic reprogramming of central cell functions and gains access to numerous carbohydrate and amino acid resources within the insect. Strikingly, a mutant lacking a phage-related holin/endolysin (HE) cassette, which is located within Tc-PAIYe, resembled the phenotypes of W22703 ΔtcaA, suggesting that this dual lysis cassette may be an example of a phage-related function that has been adapted for the release of a bacterial toxin.
Copyright: © 2022 Sänger et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Regulation and Functionality of a Holin/Endolysin Pair Involved in Killing of Galleria mellonella and Caenorhabditis elegans by Yersinia enterocolitica.Appl Environ Microbiol. 2023 Jun 28;89(6):e0003623. doi: 10.1128/aem.00036-23. Epub 2023 May 15. Appl Environ Microbiol. 2023. PMID: 37184385 Free PMC article.
-
Lon Protease- and Temperature-Dependent Activity of a Lysis Cassette Located in the Insecticidal Island of Yersinia enterocolitica.J Bacteriol. 2021 Feb 8;203(5):e00616-20. doi: 10.1128/JB.00616-20. Print 2021 Feb 8. J Bacteriol. 2021. PMID: 33288626 Free PMC article.
-
Insecticidal genes of Yersinia spp.: taxonomical distribution, contribution to toxicity towards Manduca sexta and Galleria mellonella, and evolution.BMC Microbiol. 2008 Dec 8;8:214. doi: 10.1186/1471-2180-8-214. BMC Microbiol. 2008. PMID: 19063735 Free PMC article.
-
Comparative analysis of the Photorhabdus luminescens and the Yersinia enterocolitica genomes: uncovering candidate genes involved in insect pathogenicity.BMC Genomics. 2008 Jan 25;9:40. doi: 10.1186/1471-2164-9-40. BMC Genomics. 2008. PMID: 18221513 Free PMC article. Review.
-
Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing.Virulence. 2016 Apr 2;7(3):214-29. doi: 10.1080/21505594.2015.1135289. Epub 2016 Jan 5. Virulence. 2016. PMID: 26730990 Free PMC article. Review.
Cited by
-
Yersinia entomophaga Tc toxin is released by T10SS-dependent lysis of specialized cell subpopulations.Nat Microbiol. 2024 Feb;9(2):390-404. doi: 10.1038/s41564-023-01571-z. Epub 2024 Jan 18. Nat Microbiol. 2024. PMID: 38238469 Free PMC article.
-
Uncharacterized and lineage-specific accessory genes within the Proteus mirabilis pan-genome landscape.mSystems. 2023 Aug 31;8(4):e0015923. doi: 10.1128/msystems.00159-23. Epub 2023 Jun 21. mSystems. 2023. PMID: 37341494 Free PMC article.
-
Galleria mellonella-A Model for the Study of aPDT-Prospects and Drawbacks.Microorganisms. 2023 May 31;11(6):1455. doi: 10.3390/microorganisms11061455. Microorganisms. 2023. PMID: 37374956 Free PMC article. Review.
-
Influence of the Origin, Feeding Status, and Trypanosoma cruzi Infection in the Microbial Composition of the Digestive Tract of Triatoma pallidipennis.Biology (Basel). 2025 Aug 2;14(8):984. doi: 10.3390/biology14080984. Biology (Basel). 2025. PMID: 40906147 Free PMC article.
-
Stepwise assembly and release of Tc toxins from Yersinia entomophaga.Nat Microbiol. 2024 Feb;9(2):405-420. doi: 10.1038/s41564-024-01611-2. Epub 2024 Feb 5. Nat Microbiol. 2024. PMID: 38316932 Free PMC article.
References
-
- Weber M, Fuchs TM. Metabolism in the niche: a large-scale genome-based survey reveals inositol utilization to be widespread among soil, commensal, and pathogenic bacteria. Microbiol Spectr. 2022;10(4):e0201322. Epub 2022/08/05. doi: 10.1128/spectrum.02013-22 ; PubMed Central PMCID: PMC9430895. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

