Preclinical Characterization of XL092, a Novel Receptor Tyrosine Kinase Inhibitor of MET, VEGFR2, AXL, and MER
- PMID: 36399631
- PMCID: PMC9890135
- DOI: 10.1158/1535-7163.MCT-22-0262
Preclinical Characterization of XL092, a Novel Receptor Tyrosine Kinase Inhibitor of MET, VEGFR2, AXL, and MER
Abstract
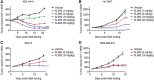
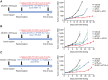
The multi-receptor tyrosine kinase inhibitor XL092 has been developed to inhibit the activity of oncogenic targets, including MET, VEGFR2, and the TAM family of kinases TYRO3, AXL and MER. Presented here is a preclinical evaluation of XL092. XL092 causes a significant decrease in tumor MET and AXL phosphorylation (P < 0.01) in murine Hs 746T xenograft models relative to vehicle, and a 96% inhibition of VEGFR2 phosphorylation in murine lungs. Dose-dependent tumor growth inhibition with XL092 was observed in various murine xenograft models, with dose-dependent tumor regression seen in the NCI-H441 model. Tumor growth inhibition was enhanced with the combination of XL092 with anti-PD-1, anti-programmed death ligand-1 (PD-L1), or anti-CTLA-4 compared with any of these agents alone in the MC38 murine syngeneic model and with anti-PD-1 in the CT26 colorectal cancer survival model. In vivo, XL092 promoted a decrease in the tumor microvasculature and significant increases of peripheral CD4+ T cells and B cells and decreases in myeloid cells versus vehicle. Significant increases in CD8+ T cells were also observed with XL092 plus anti-PD-1 or anti-PD-L1 versus vehicle. In addition, XL092 promoted M2 to M1 repolarization of macrophages in vitro and inhibited primary human macrophage efferocytosis in a dose-dependent manner. In summary, XL092 was shown to have significant antitumor and immunomodulatory activity in animal models both alone and in combination with immune checkpoint inhibitors, supporting its evaluation in clinical trials.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer 2014;14:769–85. - PubMed
-
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89–103. - PubMed
-
- Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 2004;6:61–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

