A phase 2 study of interleukin-22 and systemic corticosteroids as initial treatment for acute GVHD of the lower GI tract
- PMID: 36399701
- PMCID: PMC10163318
- DOI: 10.1182/blood.2021015111
A phase 2 study of interleukin-22 and systemic corticosteroids as initial treatment for acute GVHD of the lower GI tract
Abstract
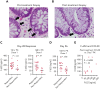
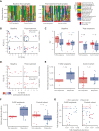
Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality following allogeneic hematopoietic transplantation. In experimental models, interleukin-22 promotes epithelial regeneration and induces innate antimicrobial molecules. We conducted a multicenter single-arm phase 2 study evaluating the safety and efficacy of a novel recombinant human interleukin-22 dimer, F-652, used in combination with systemic corticosteroids for treatment of newly diagnosed lower gastrointestinal acute GVHD. The most common adverse events were cytopenias and electrolyte abnormalities, and there were no dose-limiting toxicities. Out of 27 patients, 19 (70%; 80% confidence interval, 56%-79%) achieved a day-28 treatment response, meeting the prespecified primary endpoint. Responders exhibited a distinct fecal microbiota composition characterized by expansion of commensal anaerobes, which correlated with increased overall microbial α-diversity, suggesting improvement of GVHD-associated dysbiosis. This work demonstrates a potential approach for combining immunosuppression with tissue-supportive strategies to enhance recovery of damaged mucosa and promote microbial health in patients with gastrointestinal GVHD. This trial was registered at www.clinicaltrials.gov as NCT02406651.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: D.M.P., A.M.A., S.G., M.-A.P., and A.M.H. served as advisory board members for Evive Biotechnology (Shanghai) Ltd (formerly Generon [Shanghai] Corporation Ltd). M.R.M.v.d.B. and A.M.H. hold intellectual property related to IL-22 treatment in GVHD. A.M.H. serves in a volunteer capacity as a member of the Board of Directors of the American Society for Transplantation and Cellular Therapy (ASTCT). J. Shia serves as a consultant for Paige AI. D.M.P. serves as consultant to Kadmon/Sanofi Corporation, CareDx, Incyte and Ceramedix, and receives research funding from Incyte. M.-A.P. reports honoraria from Adicet, Allovir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma; serves on DSMBs for Cidara Therapeutics, Medigene, and Sellas Life Sciences, and the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omeros; has received institutional research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis; and serves in a volunteer capacity as a member of the Board of Directors of the ASTCT and Be The Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Cellular Immunotherapy Data Resource (CIDR) Executive Committee. M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; he has received royalties from Wolters Kluwer; has consulted, received honorarium from, or participated in advisory boards for Seres Therapeutics, Vor Biopharma, Rheos Medicines, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GlaxoSmithKline, Da Volterra, Thymofox, Garuda, Novartis (spouse), Synthekine (spouse), Beigene (spouse), Kite (spouse); has intellectual property licensing with Seres Therapeutics and Juno Therapeutics; and holds a fiduciary role on the Foundation Board of DKMS (a nonprofit organization). The remaining authors declare no competing financial interests.
Figures

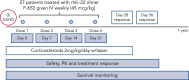
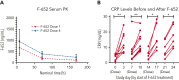
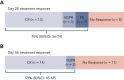
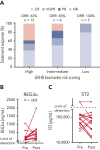
Comment in
-
IL-22, a new beacon in gastrointestinal aGVHD.Blood. 2023 Mar 23;141(12):1369-1370. doi: 10.1182/blood.2022018934. Blood. 2023. PMID: 36951884 No abstract available.
References
-
- Pasquini M, Wang Z, Horowitz MM, Gale RP. 2013 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl. 2013:187–197. - PubMed
-
- Ponce DM, Hilden P, Devlin SM, et al. High disease-free survival with enhanced protection against relapse after double-unit cord blood transplantation when compared with T cell-depleted unrelated donor transplantation in patients with acute leukemia and chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2015;21(11):1985–1993. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

