Lassa virus glycoprotein nanoparticles elicit neutralizing antibody responses and protection
- PMID: 36400021
- PMCID: PMC9794196
- DOI: 10.1016/j.chom.2022.10.018
Lassa virus glycoprotein nanoparticles elicit neutralizing antibody responses and protection
Abstract
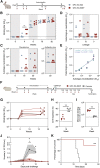
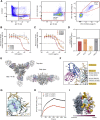
The Lassa virus is endemic in parts of West Africa, and it causes hemorrhagic fever with high mortality. The development of a recombinant protein vaccine has been hampered by the instability of soluble Lassa virus glycoprotein complex (GPC) trimers, which disassemble into monomeric subunits after expression. Here, we use two-component protein nanoparticles consisting of trimeric and pentameric subunits to stabilize GPC in a trimeric conformation. These GPC nanoparticles present twenty prefusion GPC trimers on the surface of an icosahedral particle. Cryo-EM studies of GPC nanoparticles demonstrated a well-ordered structure and yielded a high-resolution structure of an unliganded GPC. These nanoparticles induced potent humoral immune responses in rabbits and protective immunity against the lethal Lassa virus challenge in guinea pigs. Additionally, we isolated a neutralizing antibody that mapped to the putative receptor-binding site, revealing a previously undefined site of vulnerability. Collectively, these findings offer potential approaches to vaccine and therapeutic design for the Lassa virus.
Keywords: Lassa virus; antibody; challenge study; cryo-EM; nanoparticles; vaccine.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Y.W. has taken up a position at AstraZeneca; all experimental work was performed prior to this development.
Figures




References
-
- Dan-Nwafor C.C., Ipadeola O., Smout E., Ilori E., Adeyemo A., Umeokonkwo C., Nwidi D., Nwachukwu W., Ukponu W., Omabe E., et al. A cluster of nosocomial Lassa fever cases in a tertiary health facility in Nigeria: description and lessons learned, 2018. Int. J. Infect. Dis. 2019;83:88–94. doi: 10.1016/j.ijid.2019.03.030. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

