Cost-effectiveness of single, high-dose, liposomal amphotericin regimen for HIV-associated cryptococcal meningitis in five countries in sub-Saharan Africa: an economic analysis of the AMBITION-cm trial
- PMID: 36400090
- PMCID: PMC10009915
- DOI: 10.1016/S2214-109X(22)00450-8
Cost-effectiveness of single, high-dose, liposomal amphotericin regimen for HIV-associated cryptococcal meningitis in five countries in sub-Saharan Africa: an economic analysis of the AMBITION-cm trial
Abstract
Background: HIV-associated cryptococcal meningitis is a leading cause of AIDS-related mortality. The AMBITION-cm trial showed that a regimen based on a single high dose of liposomal amphotericin B deoxycholate (AmBisome group) was non-inferior to the WHO-recommended treatment of seven daily doses of amphotericin B deoxycholate (control group) and was associated with fewer adverse events. We present a five-country cost-effectiveness analysis.
Methods: The AMBITION-cm trial enrolled patients with HIV-associated cryptococcal meningitis from eight hospitals in Botswana, Malawi, South Africa, Uganda, and Zimbabwe. Taking a health service perspective, we collected country-specific unit costs and individual resource-use data per participant over the 10-week trial period, calculating mean cost per participant by group, mean cost-difference between groups, and incremental cost-effectiveness ratio per life-year saved. Non-parametric bootstrapping and scenarios analyses were performed including hypothetical real-world resource use. The trial registration number is ISRCTN72509687, and the trial has been completed.
Findings: The AMBITION-cm trial enrolled 844 participants, and 814 were included in the intention-to-treat analysis (327 from Uganda, 225 from Malawi, 107 from South Africa, 84 from Botswana, and 71 from Zimbabwe) with 407 in each group, between Jan 31, 2018, and Feb 17, 2021. Using Malawi as a representative example, mean total costs per participant were US$1369 (95% CI 1314-1424) in the AmBisome group and $1237 (1181-1293) in the control group. The incremental cost-effectiveness ratio was $128 (59-257) per life-year saved. Excluding study protocol-driven cost, using a real-world toxicity monitoring schedule, the cost per life-year saved reduced to $80 (15-275). Changes in the duration of the hospital stay and antifungal medication cost showed the greatest effect in sensitivity analyses. Results were similar across countries, with the cost per life-year saved in the real-world scenario ranging from $71 in Botswana to $121 in Uganda.
Interpretation: The AmBisome regimen was cost-effective at a low incremental cost-effectiveness ratio. The regimen might be even less costly and potentially cost-saving in real-world implementation given the lower drug-related toxicity and the potential for shorter hospital stays.
Funding: European Developing Countries Clinical Trials Partnership, Swedish International Development Cooperation Agency, Wellcome Trust and Medical Research Council, UKAID Joint Global Health Trials, and the National Institute for Health Research.
Translations: For the Chichewa, Isixhosa, Luganda, Setswana and Shona translations of the abstract see Supplementary Materials section.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests TSH was the recipient of an investigator award to his institution from Gilead Sciences; speaker fees from Pfizer and Gilead Sciences; and serves as an adviser for F2G. JNJ and GM both declare speaker fees from Gilead Sciences. All other authors declare no competing interests.
Figures


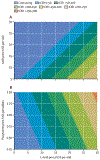
Comment in
-
Moving mycoses up the global agenda.Lancet Glob Health. 2022 Dec;10(12):e1688. doi: 10.1016/S2214-109X(22)00478-8. Lancet Glob Health. 2022. PMID: 36400070 No abstract available.
-
Cost-effectiveness of liposomal amphotericin B for HIV-associated cryptococcal meningitis.Lancet Glob Health. 2022 Dec;10(12):e1705-e1706. doi: 10.1016/S2214-109X(22)00476-4. Lancet Glob Health. 2022. PMID: 36400079 No abstract available.
References
-
- WHO. Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva: World Health Organisation, 2018. - PubMed
-
- Chen T, Mwenge L, Lakhi S, et al. Healthcare Costs and Life-years Gained From Treatments Within the Advancing Cryptococcal Meningitis Treatment for Africa (ACTA) Trial on Cryptococcal Meningitis: A Comparison of Antifungal Induction Strategies in Sub-Saharan Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2019; 69(4): 588–95. - PMC - PubMed
-
- Adler-Moore J, Lewis RE, Brüggemann RJM, Rijnders BJA, Groll AH, Walsh TJ. Preclinical Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Antifungal Activity of Liposomal Amphotericin B. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2019; 68(Suppl 4): S244–s59. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- RP-2017–08-ST2–012/DH_/Department of Health/United Kingdom
- MR/P006922/1/MRC_/Medical Research Council/United Kingdom
- K23 AI138851/AI/NIAID NIH HHS/United States
- 212638/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_MR/P006922/1/MRC_/Medical Research Council/United Kingdom
- MR/P020526/1/MRC_/Medical Research Council/United Kingdom
- 214321/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- 098316/WT_/Wellcome Trust/United Kingdom
- MR/V033417/1/MRC_/Medical Research Council/United Kingdom
- 211360/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- 203135/Z/16/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

