Prevention of Radiation-Induced Bladder Injury: A Murine Study Using Captopril
- PMID: 36400304
- PMCID: PMC12210348
- DOI: 10.1016/j.ijrobp.2022.10.033
Prevention of Radiation-Induced Bladder Injury: A Murine Study Using Captopril
Abstract
Purpose: Pelvic radiation therapy (RT) can cause debilitating bladder toxicities but few clinical interventions exist to prevent injury or alleviate symptoms. From a large genome-wide association study in patients with prostate cancer it was previously reported that SNPs tagging AGT, part of the renin-angiotensin system (RAS), correlated with patient-reported late hematuria, identifying a potential targetable pathway to prevent RT-induced bladder injury. To investigate this association, we performed a preclinical study to determine whether RAS modulation protected the bladder against RT injury.
Methods and materials: C57BL/6 male mice were treated with an oral angiotensin converting enzyme inhibitor (ACEi: 0.3g/L captopril) 5 days before focal bladder X-irradiation with either single dose (SD) 30 Gy or 3 fractions of 8 Gy (8 Gy × 3 in 5 days). RT was delivered using XStrahl SARRP Muriplan CT-image guidance with parallel-opposed lateral beams. ACEi was maintained for 20 weeks post RT. Bladder toxicity was assessed using assays to identify local injury that included urinalysis, functional micturition, bladder-released exosomes, and histopathology, as well as an assessment of systemic changes in inflammatory-mediated circulating immune cells.
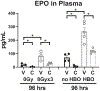
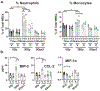
Results: SD and fractionated RT increased urinary frequency and reduced the volume of individual voids at >14 weeks, but not at 4 weeks, compared with nonirradiated animals. Urothelial layer width was positively correlated with mean volume of individual voids (P = .0428) and negatively correlated with number of voids (P = .028), relating urothelial thinning to changes in RT-mediated bladder dysfunction. These chronic RT-induced changes in micturition patterns were prevented by captopril treatment. Focal bladder irradiation significantly increased the mean particle count of urine extracellular vesicles and the monocyte and neutrophil chemokines CCL2 and MIP-2, and the proportions of circulating inflammatory-mediated neutrophils and monocytes, which was also prevented by captopril. Exploratory transcriptomic analysis of bladder tissue implicated inflammatory and erythropoietic pathways.
Conclusions: This study demonstrated that systemic modulation of the RAS protected against and alleviated RT-induced late bladder injury but larger confirmatory studies are needed.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

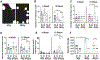
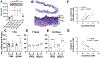
References
-
- Marks LB, Carroll PR, Dugan TC, et al. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1257–1280. - PubMed
-
- Mendenhall WM, Henderson RH, Costa JA, et al. Hemorrhagic radiation cystitis. Am J Clin Oncol 2015;38:331–336. - PubMed
-
- Pascoe C, Duncan C, Lamb BW, et al. Current management of radiation cystitis: A review and practical guide to clinical management. BJU Int 2019;123:585–594. - PubMed
-
- Oscarsson N, Muller B, Rosen A, et al. Radiation-induced cystitis treated with hyperbaric oxygen therapy (RICH-ART): A randomised, controlled, phase 2-3 trial. Lancet Oncol 2019;20:1602–1614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

