Coronavirus Disease-2019 and Implications on the Liver
- PMID: 36400465
- PMCID: PMC9385729
- DOI: 10.1016/j.cld.2022.08.003
Coronavirus Disease-2019 and Implications on the Liver
Abstract
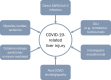
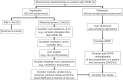
The coronavirus disease-2019 (COVID-19) pandemic has had a large impact on patients with chronic liver disease (CLD) and liver transplantation (LT) recipients. Patients with advanced CLD are at a significantly increased risk of poor outcomes in the setting of severe acute respiratory syndrome coronavirus 2 infection. The pandemic has also considerably altered the management and care that is provided to patients with CLD, pre-LT patients, and LT recipients. Vaccination against COVID-19 protects patients with CLD and LT recipients from adverse outcomes and is safe in these patients; however, vaccine efficacy may be reduced in LT recipients and other immunosuppressed patients.
Keywords: Cirrhosis; Immunosuppression; SARS-CoV-2; Transplant; Vaccination.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure The authors have no relevant commercial or financial conflicts of interest and no funding was received for this article.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

