Relative effectiveness of a 2nd booster dose of COVID-19 mRNA vaccine up to four months post administration in individuals aged 80 years or more in Italy: A retrospective matched cohort study
- PMID: 36400660
- PMCID: PMC9659513
- DOI: 10.1016/j.vaccine.2022.11.013
Relative effectiveness of a 2nd booster dose of COVID-19 mRNA vaccine up to four months post administration in individuals aged 80 years or more in Italy: A retrospective matched cohort study
Abstract
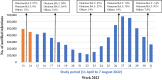
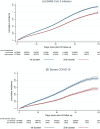
Several countries started a 2nd booster COVID-19 vaccination campaign targeting the elderly population, but evidence around its effectiveness is still scarce. This study aims to estimate the relative effectiveness of a 2nd booster dose of COVID-19 mRNA vaccine in the population aged ≥ 80 years in Italy, during predominant circulation of the Omicron BA.2 and BA.5 subvariants. We linked routine data from the national vaccination registry and the COVID-19 surveillance system. On each day between 11 April and 6 August 2022, we matched 1:1, according to several demographic and clinical characteristics, individuals who received the 2nd booster vaccine dose with individuals who received the 1st booster vaccine dose at least 120 days earlier. We used the Kaplan-Meier method to compare the risks of SARS-CoV-2 infection and severe COVID-19 (hospitalisation or death) between the two groups, calculating the relative vaccine effectiveness (RVE) as (1 - risk ratio)X100. Based on the analysis of 831,555 matched pairs, we found that a 2nd booster dose of mRNA vaccine, 14-118 days post administration, was moderately effective in preventing SARS-CoV-2 infection compared to a 1st booster dose administered at least 120 days earlier [14.3 %, 95 % confidence interval (CI): 2.2-20.2]. RVE decreased from 28.5 % (95 % CI: 24.7-32.1) in the time-interval 14-28 days to 7.6 % (95 % CI: -14.1 to 18.3) in the time-interval 56-118 days. However, RVE against severe COVID-19 was higher (34.0 %, 95 % CI: 23.4-42.7), decreasing from 43.2 % (95 % CI: 30.6-54.9) to 27.2 % (95 % CI: 8.3-42.9) over the same time span. Although RVE against SARS-CoV-2 infection was much reduced 2-4 months after a 2nd booster dose, RVE against severe COVID-19 was about 30 %, even during prevalent circulation of the Omicron BA.5 subvariant. The cost-benefit of a 3rd booster dose for the elderly people who received the 2nd booster dose at least four months earlier should be carefully evaluated.
Keywords: COVID-19; Effectiveness; Elderly population, Italy; SARS-CoV-2; mRNA vaccines.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Italian Medicines Agency (AIFA). Inserimento dell'indicazione «seconda dose booster» dei medicinali «Comirnaty» e «Spikevax» nell'elenco dei medicinali ai sensi della legge 23 dicembre 1996, n. 648. (Determina n. DG/153/2022). 11 April 2022. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto... [accessed 4 September 2022].
-
- Italian Medicines Agency (AIFA). Modifica della determina n. DG/153/2022 dell'11 aprile 2022 di inserimento dell'indicazione «seconda dose booster» dei medicinali «Comirnaty» e «Spikevax» nell'elenco dei medicinali ai sensi della legge 23 dicembre 1996, n. 648. (Determina n. DG/301/2022). (22A04093) (GU Serie Generale n.161 del 12-07-2022). 12 July 2022. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto... [accessed 4 September 2022].
-
- GitHub. Italia / Covid-19-opendata-vaccini. https://github.com/italia/covid19-opendata-vaccini/tree/master/dati [accessed 4 September 2022].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

