Single-cell profiling of vascular endothelial cells reveals progressive organ-specific vulnerabilities during obesity
- PMID: 36400935
- PMCID: PMC9684070
- DOI: 10.1038/s42255-022-00674-x
Single-cell profiling of vascular endothelial cells reveals progressive organ-specific vulnerabilities during obesity
Abstract
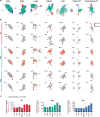
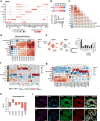
Obesity promotes diverse pathologies, including atherosclerosis and dementia, which frequently involve vascular defects and endothelial cell (EC) dysfunction. Each organ has distinct EC subtypes, but whether ECs are differentially affected by obesity is unknown. Here we use single-cell RNA sequencing to analyze transcriptomes of ~375,000 ECs from seven organs in male mice at progressive stages of obesity to identify organ-specific vulnerabilities. We find that obesity deregulates gene expression networks, including lipid handling, metabolic pathways and AP1 transcription factor and inflammatory signaling, in an organ- and EC-subtype-specific manner. The transcriptomic aberrations worsen with sustained obesity and are only partially mitigated by dietary intervention and weight loss. For example, dietary intervention substantially attenuates dysregulation of liver, but not kidney, EC transcriptomes. Through integration with human genome-wide association study data, we further identify a subset of vascular disease risk genes that are induced by obesity. Our work catalogs the impact of obesity on the endothelium, constitutes a useful resource and reveals leads for investigation as potential therapeutic targets.
© 2022. The Author(s).
Conflict of interest statement
M.B. received honoraria as a consultant and speaker from Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Lilly, Novo Nordisk, Novartis and Sanofi. All other authors declare no competing interests.
Figures















References
-
- World Health Organization, WHO Fact sheet—obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2016).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

