A new enzymatic assay to quantify inorganic pyrophosphate in plasma
- PMID: 36400967
- PMCID: PMC9839608
- DOI: 10.1007/s00216-022-04430-8
A new enzymatic assay to quantify inorganic pyrophosphate in plasma
Abstract
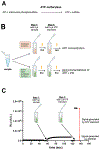
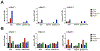
Inorganic pyrophosphate (PPi) is a crucial extracellular mineralization regulator. Low plasma PPi concentrations underlie the soft tissue calcification present in several rare hereditary mineralization disorders as well as in more common conditions like chronic kidney disease and diabetes. Even though deregulated plasma PPi homeostasis is known to be linked to multiple human diseases, there is currently no reliable assay for its quantification. We here describe a PPi assay that employs the enzyme ATP sulfurylase to convert PPi into ATP. Generated ATP is subsequently quantified by firefly luciferase-based bioluminescence. An internal ATP standard was used to correct for sample-specific interference by matrix compounds on firefly luciferase activity. The assay was validated and shows excellent precision (< 3.5%) and accuracy (93-106%) of PPi spiked into human plasma samples. We found that of several anticoagulants tested only EDTA effectively blocked conversion of ATP into PPi in plasma after blood collection. Moreover, filtration over a 300,000-Da molecular weight cut-off membrane reduced variability of plasma PPi and removed ATP present in a membrane-enclosed compartment, possibly platelets. Applied to plasma samples of wild-type and Abcc6-/- rats, an animal model with established low circulating levels of PPi, the new assay showed lower variability than the assay that was previously in routine use in our laboratory. In conclusion, we here report a new and robust assay to determine PPi concentrations in plasma, which outperforms currently available assays because of its high sensitivity, precision, and accuracy.
Keywords: ATP sulfurylase; Ectopic mineralization; Mineralization inhibitor; Plasma; Pyrophosphate; Vascular calcification.
© 2022. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Conflicts of Interest
The authors declare no competing conflict of interest
Figures


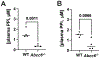
Similar articles
-
ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report.Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):1985-9. doi: 10.1161/ATVBAHA.114.304017. Epub 2014 Jun 26. Arterioscler Thromb Vasc Biol. 2014. PMID: 24969777 Free PMC article.
-
Effects of food, fasting, and exercise on plasma pyrophosphate levels and ENPP1 activity in healthy adults.Bone. 2023 Jun;171:116750. doi: 10.1016/j.bone.2023.116750. Epub 2023 Mar 30. Bone. 2023. PMID: 37003563
-
A Reference Range for Plasma Levels of Inorganic Pyrophosphate in Children Using the ATP Sulfurylase Method.J Clin Endocrinol Metab. 2022 Jan 1;107(1):109-118. doi: 10.1210/clinem/dgab615. J Clin Endocrinol Metab. 2022. PMID: 34498693 Free PMC article.
-
ABCC6, Pyrophosphate and Ectopic Calcification: Therapeutic Solutions.Int J Mol Sci. 2021 Apr 27;22(9):4555. doi: 10.3390/ijms22094555. Int J Mol Sci. 2021. PMID: 33925341 Free PMC article. Review.
-
Good-Practice Non-Radioactive Assays of Inorganic Pyrophosphatase Activities.Molecules. 2021 Apr 18;26(8):2356. doi: 10.3390/molecules26082356. Molecules. 2021. PMID: 33919593 Free PMC article. Review.
Cited by
-
Inorganic Pyrophosphate Plasma Levels Are Decreased in Pseudoxanthoma Elasticum Patients and Heterozygous Carriers but Do Not Correlate with the Genotype or Phenotype.J Clin Med. 2023 Feb 27;12(5):1893. doi: 10.3390/jcm12051893. J Clin Med. 2023. PMID: 36902680 Free PMC article.
-
Diagnosis and Treatment of Hypophosphatasia.Calcif Tissue Int. 2025 Mar 6;116(1):46. doi: 10.1007/s00223-025-01356-y. Calcif Tissue Int. 2025. PMID: 40047955 Free PMC article. Review.
-
The inorganic pyrophosphatases of microorganisms: a structural and functional review.PeerJ. 2024 Jun 24;12:e17496. doi: 10.7717/peerj.17496. eCollection 2024. PeerJ. 2024. PMID: 38938619 Free PMC article. Review.
References
-
- Jansen RS, Küçükosmanoglu A, Haas M de, Sapthu S, Otero JA, Hegman IEM, Bergen AAB, Gorgels TGMF, Borst P, Wetering K van de (2013) ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci USA 110:20206–20211. 10.1073/pnas.1319582110 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01AR072695/AR/NIAMS NIH HHS/United States
- Development/Ministry for Innovation and Technology from the source of the National Research
- R01 AR072695/AR/NIAMS NIH HHS/United States
- Bolyai János Fellowship BO/00730/19/8/Magyar Tudományos Akadémia
- Innovation Fund (ÚNKP-2022)/Ministry for Innovation and Technology from the source of the National Research
LinkOut - more resources
Full Text Sources

