Comparison of the performance of two real-time fluorescent quantitative PCR kits for the detection of SARS-CoV-2 nucleic acid: a study based on large real clinical samples
- PMID: 36401275
- PMCID: PMC9675236
- DOI: 10.1186/s12985-022-01922-y
Comparison of the performance of two real-time fluorescent quantitative PCR kits for the detection of SARS-CoV-2 nucleic acid: a study based on large real clinical samples
Abstract
Background: The global pandemic of coronavirus disease 2019 (COVID-19) has led to the development of multiple detection kits by national manufacturers for severe acute respiratory syndrome coronavirus 2 viral nucleic acid testing. The purpose of this study is to evaluate the performance of different kits (i.e., Maccura kit and Sansure kit) in real clinical work using clinical samples, which will help with the optimization of the test kits.
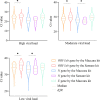
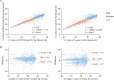
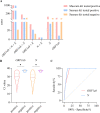
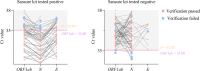
Method: During the past three months (March-May 2022), 1399 pharyngeal swabs from suspected COVID-19 patients have been initially screened using the Maccura kit in Jilin, China, and the test results were verified using the Sansure kit. The cycle threshold (Ct) values generated by the two kits were compared at different viral load levels. Correlation and consistency of the Ct values were investigated using Spearman correlation, Deming regression, and Bland-Altman plots. The cut-off Ct values of the Maccura kit were recalculated by referencing the result of the Sansure kit as a standard. Furthermore, another 163 pharyngeal swabs from suspected COVID-19 patients were collected to verify the new cut-off values.
Results: As a result of the Maccura kit testing, 1192 positive cases and 207 suspected COVID-19 cases were verified. After re-examination by the Sansure kit, 1118 positive cases were confirmed. The difference between the Ct values provided by the two kits was statistically significant, except for the N gene at high viral load. The Ct values obtained from the two kits presented a linear positive correlation. The Maccura kit used new cut-off Ct values of 35.00 (ORF1ab gene) and 35.07 (N gene). Based on that, the validation pass rate for the new cut-off Ct values was 91.41%.
Conclusion: Since the Maccura kit is found to have false positives in actual clinical work, recalculation of the cut-off values can reduce this occurrence. In order to improve the accuracy of the testing, laboratories should use two kits for COVID-19 testing, and the adjusting and optimizing of the kits for their situation are needed.
Keywords: Coronavirus disease 2019; Maccura kit; Reverse transcription-polymerase chain reaction; Sansure kit; Severe acute respiratory syndrome coronavirus 2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Performance verification of five commercial RT-qPCR diagnostic kits for SARS-CoV-2.Clin Chim Acta. 2022 Jan 15;525:46-53. doi: 10.1016/j.cca.2021.12.004. Epub 2021 Dec 10. Clin Chim Acta. 2022. PMID: 34902345 Free PMC article.
-
Comparison and Sensitivity Evaluation of Three Different Commercial Real-Time Quantitative PCR Kits for SARS-CoV-2 Detection.Viruses. 2021 Jul 8;13(7):1321. doi: 10.3390/v13071321. Viruses. 2021. PMID: 34372527 Free PMC article.
-
Comparison of the diagnostic efficacy between two PCR test kits for SARS-CoV-2 nucleic acid detection.J Clin Lab Anal. 2020 Oct;34(10):e23554. doi: 10.1002/jcla.23554. Epub 2020 Sep 25. J Clin Lab Anal. 2020. PMID: 32977349 Free PMC article.
-
Summary of the Detection Kits for SARS-CoV-2 Approved by the National Medical Products Administration of China and Their Application for Diagnosis of COVID-19.Virol Sin. 2020 Dec;35(6):699-712. doi: 10.1007/s12250-020-00331-1. Epub 2020 Dec 22. Virol Sin. 2020. PMID: 33351166 Free PMC article. Review.
-
Cross-disciplinary approaches to assist with nucleic acid testing for SARS-CoV-2.Appl Microbiol Biotechnol. 2021 Aug;105(16-17):6291-6299. doi: 10.1007/s00253-021-11498-2. Epub 2021 Aug 23. Appl Microbiol Biotechnol. 2021. PMID: 34423408 Free PMC article. Review.
Cited by
-
Performance of the Flash10 COVID-19 point-of-care molecular test.Sci Rep. 2024 Oct 27;14(1):25622. doi: 10.1038/s41598-024-77837-1. Sci Rep. 2024. PMID: 39465327 Free PMC article.
References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. 2020. https://covid19.who.int/. Accessed 3 May 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

