Integration of CRISPR/Cas9 with artificial intelligence for improved cancer therapeutics
- PMID: 36401282
- PMCID: PMC9673220
- DOI: 10.1186/s12967-022-03765-1
Integration of CRISPR/Cas9 with artificial intelligence for improved cancer therapeutics
Abstract
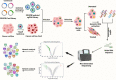
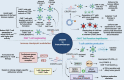
Gene editing has great potential in treating diseases caused by well-characterized molecular alterations. The introduction of clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)-based gene-editing tools has substantially improved the precision and efficiency of gene editing. The CRISPR/Cas9 system offers several advantages over the existing gene-editing approaches, such as its ability to target practically any genomic sequence, enabling the rapid development and deployment of novel CRISPR-mediated knock-out/knock-in methods. CRISPR/Cas9 has been widely used to develop cancer models, validate essential genes as druggable targets, study drug-resistance mechanisms, explore gene non-coding areas, and develop biomarkers. CRISPR gene editing can create more-effective chimeric antigen receptor (CAR)-T cells that are durable, cost-effective, and more readily available. However, further research is needed to define the CRISPR/Cas9 system's pros and cons, establish best practices, and determine social and ethical implications. This review summarizes recent CRISPR/Cas9 developments, particularly in cancer research and immunotherapy, and the potential of CRISPR/Cas9-based screening in developing cancer precision medicine and engineering models for targeted cancer therapy, highlighting the existing challenges and future directions. Lastly, we highlight the role of artificial intelligence in refining the CRISPR system's on-target and off-target effects, a critical factor for the broader application in cancer therapeutics.
Keywords: Artificial intelligence; CAR T-cells; CRISPR/Cas9; Cancer Immunotherapy; Cancer biomarker; Cancer precision medicine; Drug resistance; Epigenetics; Genome engineering.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

