Capsaicin alleviates cisplatin-induced muscle loss and atrophy in vitro and in vivo
- PMID: 36401337
- PMCID: PMC9891949
- DOI: 10.1002/jcsm.13120
Capsaicin alleviates cisplatin-induced muscle loss and atrophy in vitro and in vivo
Abstract
Background: Cisplatin (CP) is a widely used chemotherapeutic drug with subsequent adverse effects on different organs and tissues including skeletal muscle loss and atrophy as the most common clinical symptoms. The molecular mechanism of cisplatin-induced muscle atrophy is not clearly understood. However, recent significant advances indicate that it is related to an imbalance in both the protein status and apoptosis. Capsaicin (CAP) is one of the major ingredients in chilli peppers. It is a valuable pharmacological agent with several therapeutic applications in controlling pain and inflammation with particular therapeutic potential in muscle atrophy. However, the mechanisms underlying its protective effects against cisplatin-induced muscle loss and atrophy remain largely unknown. This study aims to investigate capsaicin's beneficial effects on cisplatin-induced muscle loss and atrophy in vitro and in vivo.
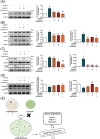
Methods: The anti-muscle-atrophic effect of capsaicin on cisplatin-induced muscle loss was investigated using in vivo and in vitro studies. By using the pretreatment model, pretreated capsaicin for 24 h and treated with cisplatin for 48 h, we utilized a C2 C12 myotube formation model where cell viability analysis, immunofluorescence, and protein expression were measured to investigate the effect of capsaicin in hampering cisplatin-induced muscle atrophy. C57BL/6 mice were administered capsaicin (10, 40 mg/kg BW) as a pretreatment for 5 weeks and cisplatin (3 mg/kg BW) for seven consecutively days to assess muscle atrophy in an animal model for protein and oxidative stress examination, and the grip strength was tested to evaluate the muscle strength.
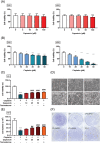
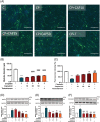
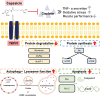
Results: Our study results indicated that cisplatin caused lower cell viability and showed a subset of hallmark signs typically recognized during atrophy, including severe reduction in the myotube diameter, repression of Akt, and mTOR protein expression. However, pretreatment with capsaicin could ameliorate cisplatin-induced muscle atrophy by up-regulating the protein synthesis in skeletal muscle as well as down-regulating the markers of protein degradation. Additionally, capsaicin was able to downregulate the protein expression of apoptosis-related markers, activated TRPV1 and autophagy progress modulation and the recovery of lysosome function. In vivo, capsaicin could relieve oxidative stress and cytokine secretion while modulating autophagy-related lysosome fusion, improving grip strength, and alleviating cisplatin-induced body weight loss and gastrocnemius atrophy.
Conclusions: These findings suggest that capsaicin can restore cisplatin-induced imbalance between protein synthesis and protein degradation pathways and it may have protective effects against cisplatin-induced muscle atrophy.
Keywords: Capsaicin; Cisplatin; Muscle atrophy; Myotube.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep 2003;10:1663–1682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

