Role of renal tubular programed cell death in diabetic kidney disease
- PMID: 36401596
- PMCID: PMC10078574
- DOI: 10.1002/dmrr.3596
Role of renal tubular programed cell death in diabetic kidney disease
Abstract
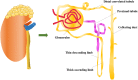
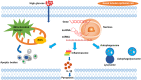
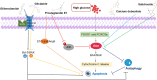
The pathogenic mechanism of diabetic kidney disease (DKD) is involved in various functions; however, its inadequate characterisation limits the availability of effective treatments. Tubular damage is closely correlated with renal function and is thought to be the main contributor to the injury observed in early DKD. Programed cell death (PCD) occurs during the biological development of the living body. Accumulating evidence has clarified the fundamental role of abnormalities in tubular PCD during DKD pathogenesis. Among PCD types, classical apoptosis, autophagic cell death, and pyroptosis are the most studied and will be the focus of this review. Our review aims to elucidate the current knowledge of the mechanism of DKD and the potential therapeutic potential of drugs targeting tubular PCD pathways in DKD.
Keywords: apoptosis; autophagy; diabetic kidney disease; programed cell death; pyroptosis; review.
© 2022 The Authors. Diabetes/Metabolism Research and Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Milas O, Gadalean F, Vlad A, et al. Pro‐inflammatory cytokines are associated with podocyte damage and proximal tubular dysfunction in the early stage of diabetic kidney disease in type 2 diabetes mellitus patients. J Diabetes Complications. 2020;34(2):107479. 10.1016/j.jdiacomp.2019.107479 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

