Unsupervised physiological noise correction of functional magnetic resonance imaging data using phase and magnitude information (PREPAIR)
- PMID: 36401844
- PMCID: PMC9875918
- DOI: 10.1002/hbm.26152
Unsupervised physiological noise correction of functional magnetic resonance imaging data using phase and magnitude information (PREPAIR)
Abstract
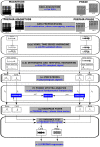
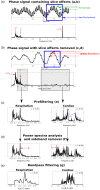
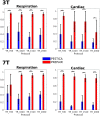
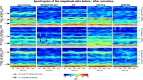
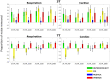
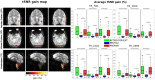
Of the sources of noise affecting blood oxygen level-dependent functional magnetic resonance imaging (fMRI), respiration and cardiac fluctuations are responsible for the largest part of the variance, particularly at high and ultrahigh field. Existing approaches to removing physiological noise either use external recordings, which can be unwieldy and unreliable, or attempt to identify physiological noise from the magnitude fMRI data. Data-driven approaches are limited by sensitivity, temporal aliasing, and the need for user interaction. In the light of the sensitivity of the phase of the MR signal to local changes in the field stemming from physiological processes, we have developed an unsupervised physiological noise correction method using the information carried in the phase and the magnitude of echo-planar imaging data. Our technique, Physiological Regressor Estimation from Phase and mAgnItude, sub-tR (PREPAIR) derives time series signals sampled at the slice TR from both phase and magnitude images. It allows physiological noise to be captured without aliasing, and efficiently removes other sources of signal fluctuations not related to physiology, prior to regressor estimation. We demonstrate that the physiological signal time courses identified with PREPAIR agree well with those from external devices and retrieve challenging cardiac dynamics. The removal of physiological noise was as effective as that achieved with the most used approach based on external recordings, RETROICOR. In comparison with widely used recording-free physiological noise correction tools-PESTICA and FIX, both performed in unsupervised mode-PREPAIR removed significantly more respiratory and cardiac noise than PESTICA, and achieved a larger increase in temporal signal-to-noise-ratio at both 3 and 7 T.
Keywords: PREPAIR; fMRI; noise correction; phase data; physiological noise; unsupervised.
© 2022 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
Similar articles
-
Phase vs. magnitude information in functional magnetic resonance imaging time series: toward understanding the noise.Magn Reson Imaging. 2009 Oct;27(8):1046-57. doi: 10.1016/j.mri.2009.02.006. Epub 2009 Apr 15. Magn Reson Imaging. 2009. PMID: 19369024
-
A comprehensive investigation of physiologic noise modeling in resting state fMRI; time shifted cardiac noise in EPI and its removal without external physiologic signal measures.Neuroimage. 2022 Jul 1;254:119136. doi: 10.1016/j.neuroimage.2022.119136. Epub 2022 Mar 26. Neuroimage. 2022. PMID: 35346840
-
Phase stability in fMRI time series: effect of noise regression, off-resonance correction and spatial filtering techniques.Neuroimage. 2012 Feb 15;59(4):3748-61. doi: 10.1016/j.neuroimage.2011.10.095. Epub 2011 Nov 4. Neuroimage. 2012. PMID: 22079450
-
The effect of physiological noise in phase functional magnetic resonance imaging: from blood oxygen level-dependent effects to direct detection of neuronal currents.Magn Reson Imaging. 2008 Sep;26(7):1026-40. doi: 10.1016/j.mri.2008.01.010. Epub 2008 May 13. Magn Reson Imaging. 2008. PMID: 18479875 Review.
-
Physiological Confounds in BOLD-fMRI and Their Correction.NMR Biomed. 2025 Jul;38(7):e70076. doi: 10.1002/nbm.70076. NMR Biomed. 2025. PMID: 40491186 Free PMC article. Review.
Cited by
-
Comparing data-driven physiological denoising approaches for resting-state fMRI: implications for the study of aging.Front Neurosci. 2024 Feb 6;18:1223230. doi: 10.3389/fnins.2024.1223230. eCollection 2024. Front Neurosci. 2024. PMID: 38379761 Free PMC article.
-
Detection of respiration-induced field modulations in fMRI: A concurrent and navigator-free approach.Imaging Neurosci (Camb). 2024 Feb 12;2:imag-2-00091. doi: 10.1162/imag_a_00091. eCollection 2024. Imaging Neurosci (Camb). 2024. PMID: 40800327 Free PMC article.
-
Improved dynamic distortion correction for fMRI using single-echo EPI and a readout-reversed first image (REFILL).Hum Brain Mapp. 2023 Oct 15;44(15):5095-5112. doi: 10.1002/hbm.26440. Epub 2023 Aug 7. Hum Brain Mapp. 2023. PMID: 37548414 Free PMC article.
References
-
- Ahn, G. B. , Chi, C. S. , & Chuang, C. S. (1991). Recording of cerebral blood flow velocity using transcranial doppler ultrasound in Normal subjects. Journal of the Korean Neurological Association, 9, 277–285.
-
- Bachrata, B. , Eckstein, K. , Trattnig, S. , & Robinson, S. D. (2018). Considerations in quantitative susceptibility mapping using echo‐planar imaging. Proceedings of the 26th Annual Meeting of ISMRM; Paris, France. Paris, France #4996.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

