Oral tolerance to prevent anti-drug antibody formation in protein replacement therapies
- PMID: 36402002
- PMCID: PMC9730862
- DOI: 10.1016/j.cellimm.2022.104641
Oral tolerance to prevent anti-drug antibody formation in protein replacement therapies
Abstract
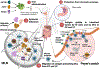
Protein based therapeutics have successfully improved the quality of life for patients of monogenic disorders like hemophilia, Pompe and Fabry disease. However, a significant proportion of patients develop immune responses towards intravenously infused therapeutic protein, which can complicate or neutralize treatment and compromise patient safety. Strategies aimed at circumventing immune responses following therapeutic protein infusion can greatly improve therapeutic efficacy. In recent years, antigen-based oral tolerance induction has shown promising results in the prevention and treatment of autoimmune diseases, food allergies and can prevent anti-drug antibody formation to protein replacement therapies. Oral tolerance exploits regulatory mechanisms that are initiated in the gut associated lymphoid tissue (GALT) to promote active suppression of orally ingested antigen. In this review, we outline general perceptions and current knowledge about the mechanisms of oral tolerance, including tissue specific sites of tolerance induction and the cells involved, with emphasis on antigen presenting cells and regulatory T cells. We define several factors, such as cytokines and metabolites that impact the stability and expansion potential of these immune modulatory cells. We highlight preclinical studies that have been performed to induce oral tolerance to therapeutic proteins or enzymes for single gene disorders, such as hemophilia or Pompe disease. These studies mainly utilize a transgenic plant-based system for oral delivery of antigen in conjugation with fusion protein technology that favors the prevention of antigen degradation in the stomach while enhancing uptake in the small intestine by antigen presenting cells and regulatory T cell induction, thereby promoting antigen specific systemic tolerance.
Keywords: Anti-drug antibody; Enzyme replacement therapy; FIX; FVIII; Hemophilia; IL-10; LAP; Oral tolerance; Pompe disease; Tregs.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Suppression of anti-drug antibody formation against coagulation factor VIII by oral delivery of anti-CD3 monoclonal antibody in hemophilia A mice.Cell Immunol. 2023 Mar;385:104675. doi: 10.1016/j.cellimm.2023.104675. Epub 2023 Jan 30. Cell Immunol. 2023. PMID: 36746071 Free PMC article.
-
Plant cell-made protein antigens for induction of Oral tolerance.Biotechnol Adv. 2019 Nov 15;37(7):107413. doi: 10.1016/j.biotechadv.2019.06.012. Epub 2019 Jun 26. Biotechnol Adv. 2019. PMID: 31251968 Free PMC article. Review.
-
Antigen-specific in vitro expansion of factor VIII-specific regulatory T cells induces tolerance in hemophilia A mice.J Thromb Haemost. 2020 Feb;18(2):328-340. doi: 10.1111/jth.14659. Epub 2019 Oct 29. J Thromb Haemost. 2020. PMID: 31609041 Free PMC article.
-
Tolerating Factor VIII: Recent Progress.Front Immunol. 2020 Jan 10;10:2991. doi: 10.3389/fimmu.2019.02991. eCollection 2019. Front Immunol. 2020. PMID: 31998296 Free PMC article. Review.
-
Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells.Blood. 2015 Apr 9;125(15):2418-27. doi: 10.1182/blood-2014-08-597070. Epub 2015 Feb 19. Blood. 2015. PMID: 25700434 Free PMC article.
Cited by
-
Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice.Int J Mol Sci. 2024 Aug 9;25(16):8701. doi: 10.3390/ijms25168701. Int J Mol Sci. 2024. PMID: 39201385 Free PMC article.
-
Anti-Drug Antibody Response to Therapeutic Antibodies and Potential Mitigation Strategies.Biomedicines. 2025 Jan 26;13(2):299. doi: 10.3390/biomedicines13020299. Biomedicines. 2025. PMID: 40002712 Free PMC article. Review.
-
Potential role for oral tolerance in gene therapy.Cell Immunol. 2023 Sep-Oct;391-392:104742. doi: 10.1016/j.cellimm.2023.104742. Epub 2023 Jun 28. Cell Immunol. 2023. PMID: 37423874 Free PMC article.
-
Distinct functions and transcriptional signatures in orally induced regulatory T cell populations.Front Immunol. 2023 Oct 26;14:1278184. doi: 10.3389/fimmu.2023.1278184. eCollection 2023. Front Immunol. 2023. PMID: 37954612 Free PMC article.
-
Progress and Challenges in the Treatment of Fabry Disease.BioDrugs. 2025 Jul;39(4):517-535. doi: 10.1007/s40259-025-00723-3. Epub 2025 May 1. BioDrugs. 2025. PMID: 40310476 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

