Monitoring leaching of Cd2+ from cadmium-based quantum dots by an Cd aptamer fluorescence sensor
- PMID: 36402100
- PMCID: PMC10139768
- DOI: 10.1016/j.bios.2022.114880
Monitoring leaching of Cd2+ from cadmium-based quantum dots by an Cd aptamer fluorescence sensor
Abstract
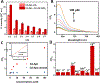
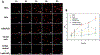
Quantum Dots (QDs) have been demonstrated with outstanding optical properties and thus been widely used in many biological and biomedical studies. However, previous studies have shown that QDs can cause cell toxicity, mainly attributable to the leached Cd2+. Therefore, identifying the leaching kinetics is very important to understand QD biosafety and cytotoxicity. Toward this goal, instrumental analyses such as inductively coupled plasma mass spectrometry (ICP-MS) have been used, which are time-consuming, costly and do not provide real-time or spatial information. To overcome these limitations, we report herein a fast and cost-effective fluorescence sensor based a Cd2+-specific aptamer for real-time monitoring the rapid leaching kinetics of QDs in vitro and in living cells. The sensor shows high specificity towards Cd2+ and is able to measure the Cd2+ leached either from water-dispersed CdTe QDs or two-layered CdSe/CdS QDs. The sensor is then used to study the stability of these two types of QDs under conditions to mimic cellular pH and temperature and the results from the sensor are similar to those obtained from ICP-MS. Finally, the sensor is able to monitor the leaching of Cd2+ from QDs in HeLa cells. The fluorescence aptamer sensor described in this study may find many applications as a tool for understanding biosafety of numerous other Cd-based QDs, including leaching kinetics and toxicity mechanisms in living systems.
Keywords: Aptamer sensor; Cadmium-based quantum dots; Real-time monitoring.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Degradation of aqueous synthesized CdTe/ZnS quantum dots in mice: differential blood kinetics and biodistribution of cadmium and tellurium.Part Fibre Toxicol. 2013 Aug 6;10:37. doi: 10.1186/1743-8977-10-37. Part Fibre Toxicol. 2013. PMID: 23915017 Free PMC article.
-
[Size exclusionchromatography-high-performance liquid chromatography-inductively coupled plasma mass spectrometry for measuring the stability of cadmium telluridequantum dots].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017 Mar 20;35(3):217-220. doi: 10.3760/cma.j.issn.1001-9391.2017.03.016. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017. PMID: 28511312 Chinese.
-
Detail investigation of toxicity, bioaccumulation, and translocation of Cd-based quantum dots and Cd salt in white mustard.Chemosphere. 2020 Jul;251:126174. doi: 10.1016/j.chemosphere.2020.126174. Epub 2020 Feb 12. Chemosphere. 2020. PMID: 32151804
-
Progress on the toxicity of quantum dots to model organism-zebrafish.J Appl Toxicol. 2023 Jan;43(1):89-106. doi: 10.1002/jat.4333. Epub 2022 May 5. J Appl Toxicol. 2023. PMID: 35441386 Review.
-
The cytotoxicity of cadmium-based quantum dots.Biomaterials. 2012 Feb;33(5):1238-44. doi: 10.1016/j.biomaterials.2011.10.070. Epub 2011 Nov 10. Biomaterials. 2012. PMID: 22078811 Review.
Cited by
-
A Review of in vivo Toxicity of Quantum Dots in Animal Models.Int J Nanomedicine. 2023 Dec 29;18:8143-8168. doi: 10.2147/IJN.S434842. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38170122 Free PMC article. Review.
-
Current research status of tumor cell biomarker detection.Microsyst Nanoeng. 2023 Oct 5;9:123. doi: 10.1038/s41378-023-00581-5. eCollection 2023. Microsyst Nanoeng. 2023. PMID: 37811123 Free PMC article. Review.
-
Genetically Encoded Fluorogenic DNA Aptamers for Imaging Metabolite in Living Cells.J Am Chem Soc. 2025 Jan 15;147(2):1529-1541. doi: 10.1021/jacs.4c09855. Epub 2024 Dec 31. J Am Chem Soc. 2025. PMID: 39739942 Free PMC article.
-
Stimuli-responsive smart materials enabled high-performance biosensors for liquid biopsies.J Nanobiotechnology. 2025 Jul 1;23(1):477. doi: 10.1186/s12951-025-03541-5. J Nanobiotechnology. 2025. PMID: 40597218 Free PMC article. Review.
-
Enrofloxacin Rapid Detection in Aquatic Foods: Based on DNA Aptamer Sensor.Foods. 2024 Mar 20;13(6):941. doi: 10.3390/foods13060941. Foods. 2024. PMID: 38540931 Free PMC article.
References
-
- Ai J, Li T, Li B, Xu Y, Li D, Liu Z, Wang E, 2012. Anal. Chim. Acta, 741, 93–99. - PubMed
-
- Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H, Spiccia L, 2011. Adv. Mater, 23(12), H18–40. - PubMed
-
- Billet B, Chovelon B, Fiore E, Faure P, Ravelet C, Peyrin E, 2022. Biosens. Bioelectron, 205, 114091. - PubMed
-
- Bruchez MJ., Moronne M., Gin P., Welss S., Alivisatos AP., 1998. Science, 281(5385), 2013–2016. - PubMed
-
- Cao Q, Li J, Wang E, 2019. Biosens. Bioelectron, 132, 333–342. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

