A Zika virus-specific IgM elicited in pregnancy exhibits ultrapotent neutralization
- PMID: 36402135
- PMCID: PMC9742325
- DOI: 10.1016/j.cell.2022.10.023
A Zika virus-specific IgM elicited in pregnancy exhibits ultrapotent neutralization
Abstract
Congenital Zika virus (ZIKV) infection results in neurodevelopmental deficits in up to 14% of infants born to ZIKV-infected mothers. Neutralizing antibodies are a critical component of protective immunity. Here, we demonstrate that plasma IgM contributes to ZIKV immunity in pregnancy, mediating neutralization up to 3 months post-symptoms. From a ZIKV-infected pregnant woman, we isolated a pentameric ZIKV-specific IgM (DH1017.IgM) that exhibited ultrapotent ZIKV neutralization dependent on the IgM isotype. DH1017.IgM targets an envelope dimer epitope within domain II. The epitope arrangement on the virion is compatible with concurrent engagement of all ten antigen-binding sites of DH1017.IgM, a solution not available to IgG. DH1017.IgM protected mice against viremia upon lethal ZIKV challenge more efficiently than when expressed as an IgG. Our findings identify a role for antibodies of the IgM isotype in protection against ZIKV and posit DH1017.IgM as a safe and effective candidate immunotherapeutic, particularly during pregnancy.
Keywords: IgM; Zika; cross-linking; immunotherapy; isotype; multivalent binding; neutralization; pregnancy; therapeutic antibodies.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests M.B., S.R.P., T.S., and K.-K.H. have filed a patent application directed to antibodies that are related to this work. S.R.P. serves as a consultant to Moderna, Merck, Pfizer, GSK, Hoopika, and Dynavax CMV vaccine programs and leads a sponsored research program with Moderna and Merck.
Figures
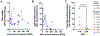
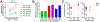
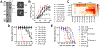



Comment in
-
Neutralizing Zika virus.Nat Rev Drug Discov. 2023 Jan;22(1):19. doi: 10.1038/d41573-022-00201-3. Nat Rev Drug Discov. 2023. PMID: 36450861 No abstract available.
References
-
- Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, et al. (2016). Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 353, 1129–1132. 10.1126/science.aah6157. - DOI - PMC - PubMed
-
- Bonsignori M, Hwang K-K, Chen X, Tsao C-Y, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, et al. (2011). Analysis of a Clonal Lineage of HIV-1 Envelope V2/V3 Conformational Epitope-Specific Broadly Neutralizing Antibodies and Their Inferred Unmutated Common Ancestors. J. Virol 85, 9998–10009. 10.1128/JVI.05045-11. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

