Design of a pragmatic clinical trial to improve screening and treatment for opioid use disorder in primary care
- PMID: 36402275
- PMCID: PMC9839646
- DOI: 10.1016/j.cct.2022.107012
Design of a pragmatic clinical trial to improve screening and treatment for opioid use disorder in primary care
Abstract
Background: Opioid-related deaths continue to rise in the U.S. A shared decision-making (SDM) system to help primary care clinicians (PCCs) identify and treat patients with opioid use disorder (OUD) could help address this crisis.
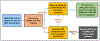
Methods: In this cluster-randomized trial, primary care clinics in three healthcare systems were randomized to receive or not receive access to an OUD-SDM system. The OUD-SDM system alerts PCCs and patients to elevated risk of OUD and supports OUD screening and treatment. It includes guidance on OUD screening and diagnosis, treatment selection, starting and maintaining patients on buprenorphine for waivered clinicians, and screening for common comorbid conditions. The primary study outcome is, of patients at high risk for OUD, the percentage receiving an OUD diagnosis within 30 days of index visit. Additional outcomes are, of patients at high risk for or with a diagnosis of OUD, (a) the percentage receiving a naloxone prescription, or (b) the percentage receiving a medication for OUD (MOUD) prescription or referral to specialty care within 30 days of an index visit, and (c) total days covered by a MOUD prescription within 90 days of an index visit.
Results: The intervention started in April 2021 and continues through December 2023. PCCs and patients in 90 clinics are included; study results are expected in 2024.
Conclusion: This protocol paper describes the design of a multi-site trial to help PCCs recognize and treat OUD. If effective, this OUD-SDM intervention could improve screening of at-risk patients and rates of OUD treatment for people with OUD.
Keywords: Opioid overdose; Opioid use disorder; Pragmatic clinical trial; Primary care; Shared decision-making.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
A randomized controlled trial of an intervention to reduce stigma toward people with opioid use disorder among primary care clinicians.Addict Sci Clin Pract. 2023 Feb 11;18(1):10. doi: 10.1186/s13722-023-00366-1. Addict Sci Clin Pract. 2023. PMID: 36774521 Free PMC article. Clinical Trial.
-
Opioid Overdose After Medication for Opioid Use Disorder Initiation Following Hospitalization or ED Visit.JAMA Netw Open. 2024 Jul 1;7(7):e2423954. doi: 10.1001/jamanetworkopen.2024.23954. JAMA Netw Open. 2024. PMID: 39037812 Free PMC article.
-
Are North Carolina clinicians delivering opioid use disorder treatment to Medicaid beneficiaries?Addiction. 2022 Nov;117(11):2855-2863. doi: 10.1111/add.15854. Epub 2022 Mar 7. Addiction. 2022. PMID: 35194878 Free PMC article.
-
Caring for Hospitalized Adults With Opioid Use Disorder in the Era of Fentanyl: A Review.JAMA Intern Med. 2024 Jun 1;184(6):691-701. doi: 10.1001/jamainternmed.2023.7282. JAMA Intern Med. 2024. PMID: 38683591 Review.
-
Management of opioid withdrawal and initiation of medications for opioid use disorder in the hospital setting.Hosp Pract (1995). 2022 Oct;50(4):251-258. doi: 10.1080/21548331.2022.2102776. Epub 2022 Jul 22. Hosp Pract (1995). 2022. PMID: 35837678 Review.
Cited by
-
Randomized Pilot of a Clinical Decision Support Tool to Increase Suicide Screening for at-Risk Primary Care Patients With Opioid Use Disorder.AJPM Focus. 2024 Oct 2;3(6):100280. doi: 10.1016/j.focus.2024.100280. eCollection 2024 Dec. AJPM Focus. 2024. PMID: 39605784 Free PMC article.
-
Clinical Decision Support and Cardiometabolic Medication Adherence: A Randomized Clinical Trial.JAMA Netw Open. 2025 Jan 2;8(1):e2453745. doi: 10.1001/jamanetworkopen.2024.53745. JAMA Netw Open. 2025. PMID: 39786775 Free PMC article. Clinical Trial.
-
A randomized controlled trial of an intervention to reduce stigma toward people with opioid use disorder among primary care clinicians.Addict Sci Clin Pract. 2023 Feb 11;18(1):10. doi: 10.1186/s13722-023-00366-1. Addict Sci Clin Pract. 2023. PMID: 36774521 Free PMC article. Clinical Trial.
-
'Do they care?': a qualitative examination of patient perspectives on primary care clinician communication related to opioids in the USA.BMJ Open. 2025 Jan 7;15(1):e090462. doi: 10.1136/bmjopen-2024-090462. BMJ Open. 2025. PMID: 39773800 Free PMC article.
-
Barriers and Facilitators to Using a Clinical Decision Support Tool for Opioid Use Disorder in Primary Care.J Am Board Fam Med. 2024 Aug 14;37(3):389-398. doi: 10.3122/jabfm.2023.230308R1. J Am Board Fam Med. 2024. PMID: 38942448 Free PMC article.
References
-
- Drug Overdose Deaths in the U.S. Top 100,000 Annually. 2021. [cited 2022 May 22]; Available from: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm.
-
- Treatment episode data set - admissions (TEDS-A) 2012. [cited 2018 May 15]; Available from: http://wwwdasis.samhsa.gov/webt/quicklink/US09.htm.
-
- Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. 2020, Substance Abuse and Mental Health Services Administration: Rockville, MD.
-
- Substance Abuse and Mental Health Services Administration, Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63 Publication No. PEP21-02-01-002. 2021, Rockville, MD: Substance Abuse and Mental Health Services Administration.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

