Characterization and Function of Cryopreserved Bone Marrow from Deceased Organ Donors: A Potential Viable Alternative Graft Source
- PMID: 36402456
- PMCID: PMC9918674
- DOI: 10.1016/j.jtct.2022.11.010
Characterization and Function of Cryopreserved Bone Marrow from Deceased Organ Donors: A Potential Viable Alternative Graft Source
Abstract
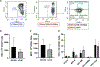
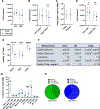
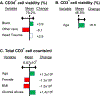
Despite the readily available graft sources for allogeneic hematopoietic cell transplantation (alloHCT), a significant unmet need remains in the timely provision of suitable unrelated donor grafts. This shortage is related to the rarity of certain HLA alleles in the donor pool, nonclearance of donors owing to infectious disease or general health status, and prolonged graft procurement and processing times. An alternative hematopoietic progenitor cell (HPC) graft source obtained from the vertebral bodies (VBs) of deceased organ donors could alleviate many of the obstacles associated with using grafts from healthy living donors or umbilical cord blood (UCB). Deceased organ donor-derived bone marrow (BM) can be preemptively screened, cryogenically banked for on-demand use, and made available in adequate cell doses for HCT. We have developed a good manufacturing practice (GMP)-compliant process to recover and cryogenically bank VB-derived HPCs from deceased organ donor (OD) BM. Here we present results from an analysis of HPCs from BM obtained from 250 deceased donors to identify any substantial difference in composition or quality compared with HPCs from BM aspirated from the iliac crests of healthy living donors. BM from deceased donor VBs was processed in a central GMP facility and packaged for cryopreservation in 5% DMSO/2.5% human serum albumin. BM aspirated from living donor iliac crests was obtained and used for comparison. A portion of each specimen was analyzed before and after cryopreservation by flow cytometry and colony-forming unit potential. Bone marrow chimerism potential was assessed in irradiated immunocompromised NSG mice. Analysis of variance with Bonferroni correction for multiple comparisons was used to determine how cryopreservation affects BM cells and to evaluate indicators of successful engraftment of BM cells into irradiated murine models. The t test (with 95% confidence intervals [CIs]) was used to compare cells from deceased donors and living donors. A final dataset of complete clinical and matched laboratory data from 226 cryopreserved samples was used in linear regressions to predict outcomes of BM HPC processing. When compared before and after cryopreservation, OD-derived BM HPCs were found to be stable, with CD34+ cells maintaining high viability and function after thawing. The yield from a single donor is sufficient for transplantation of an average of 1.6 patients (range, 1.2 to 7.5). CD34+ cells from OD-derived HPCs from BM productively engrafted sublethally irradiated immunocompromised mouse BM (>44% and >67% chimerism at 8 and 16 weeks, respectively). Flow cytometry and secondary transplantation confirmed that OD HPCs from BM is composed of long-term engrafting CD34+CD38-CD45RA-CD90+CD49f+ HSCs. Linear regression identified no meaningful predictive associations between selected donor-related characteristics and OD BM HPC quality or yield. Collectively, these data demonstrate that cryopreserved BM HPCs from deceased organ donors is potent and functionally equivalent to living donor BM HPCs and is a viable on-demand graft source for clinical HCT. Prospective clinical trials will soon commence in collaboration with the Center for International Blood and Marrow Research to assess the feasibility, safety, and efficacy of Ossium HPCs from BM (ClinicalTrials.gov identifier NCT05068401).
Keywords: Bone Marrow; CD34; Cryopreservation; Murine Engraftment; Organ Donor; Vertebral Body.
Copyright © 2022 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures
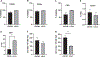

Similar articles
-
A Large-Scale Bank of Organ Donor Bone Marrow and Matched Mesenchymal Stem Cells for Promoting Immunomodulation and Transplant Tolerance.Front Immunol. 2021 Feb 26;12:622604. doi: 10.3389/fimmu.2021.622604. eCollection 2021. Front Immunol. 2021. PMID: 33732244 Free PMC article. Review.
-
Cryopreservation of Allogeneic Hematopoietic Cell Products During COVID-19 Pandemic: Graft Characterization and Engraftment Outcomes.Transplant Proc. 2023 Oct;55(8):1799-1809. doi: 10.1016/j.transproceed.2023.03.070. Epub 2023 Apr 21. Transplant Proc. 2023. PMID: 37210273 Free PMC article.
-
Universal Engraftment after Allogeneic Hematopoietic Cell Transplantation Using Cryopreserved CD34-Selected Grafts.Transplant Cell Ther. 2021 Aug;27(8):697.e1-697.e5. doi: 10.1016/j.jtct.2021.04.026. Epub 2021 May 13. Transplant Cell Ther. 2021. PMID: 33991721 Free PMC article.
-
The Effect of Donor Graft Cryopreservation on Allogeneic Hematopoietic Cell Transplantation Outcomes: A Center for International Blood and Marrow Transplant Research Analysis. Implications during the COVID-19 Pandemic.Transplant Cell Ther. 2021 Jun;27(6):507-516. doi: 10.1016/j.jtct.2021.03.015. Epub 2021 Mar 22. Transplant Cell Ther. 2021. PMID: 33865804 Free PMC article.
-
[Umbilical cord blood as a source of stem cells].Acta Med Croatica. 2006 Jun;60(3):215-25. Acta Med Croatica. 2006. PMID: 16933834 Review. Croatian.
Cited by
-
Early Cytomegalovirus Reactivation in Renal Recipients Is Associated with High Levels of B Cell Maturation Antigen Transcript Expression Prior to Transplantation.Int J Mol Sci. 2023 Jun 22;24(13):10491. doi: 10.3390/ijms241310491. Int J Mol Sci. 2023. PMID: 37445668 Free PMC article.
-
Unraveling the Enigma of Organismal Death: Insights, Implications, and Unexplored Frontiers.Physiology (Bethesda). 2024 Sep 1;39(5):0. doi: 10.1152/physiol.00004.2024. Epub 2024 Apr 16. Physiology (Bethesda). 2024. PMID: 38624244 Review.
-
Advanced Medical Countermeasures and Devices for Use During a Radiological or Nuclear Emergency.Disaster Med Public Health Prep. 2025 Jul 21;19:e199. doi: 10.1017/dmp.2025.12. Disaster Med Public Health Prep. 2025. PMID: 40686053 Review.
References
-
- Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2021. Available at: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages.... Accessed XXX.
-
- Dehn J, Chitphakdithai P, Shaw BE, et al. Likelihood of proceeding to allogeneic hematopoietic cell transplantation in the United States after search activation in the National Registry: impact of patient age, disease, and search prognosis. Transplant Cell Ther. 2021;27. 184.e1–184.e13. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

