The transcription factor OsWRKY10 inhibits phosphate uptake via suppressing OsPHT1;2 expression under phosphate-replete conditions in rice
- PMID: 36402551
- PMCID: PMC9899414
- DOI: 10.1093/jxb/erac456
The transcription factor OsWRKY10 inhibits phosphate uptake via suppressing OsPHT1;2 expression under phosphate-replete conditions in rice
Abstract
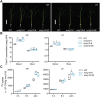
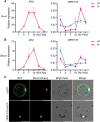
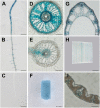
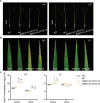
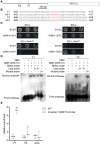
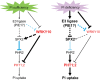
Plants have evolved delicate systems for stimulating or inhibiting inorganic phosphate (Pi) uptake in response to the fluctuating Pi availability in soil. However, the negative regulators inhibiting Pi uptake at the transcriptional level are largely unexplored. Here, we functionally characterized a transcription factor in rice (Oryza sativa), OsWRKY10. OsWRKY10 encodes a nucleus-localized protein and showed preferential tissue localization. Knockout of OsWRKY10 led to increased Pi uptake and accumulation under Pi-replete conditions. In accordance with this phenotype, OsWRKY10 was transcriptionally induced by Pi, and a subset of PHOSPHATE TRANSPORTER 1 (PHT1) genes were up-regulated upon its mutation, suggesting that OsWRKY10 is a transcriptional repressor of Pi uptake. Moreover, rice plants expressing the OsWRKY10-VP16 fusion protein (a dominant transcriptional activator) accumulated even more Pi than oswrky10. Several lines of biochemical evidence demonstrated that OsWRKY10 directly suppressed OsPHT1;2 expression. Genetic analysis showed that OsPHT1;2 was responsible for the increased Pi accumulation in oswrky10. Furthermore, during Pi starvation, OsWRKY10 protein was degraded through the 26S proteasome. Altogether, the OsWRKY10-OsPHT1;2 module represents a crucial loop in the Pi signaling network in rice, inhibiting Pi uptake when there is ample Pi in the environment.
Keywords: Negative regulation; WRKY transcription factor; phosphate signaling; phosphate transporter; phosphate uptake; phosphorus; rice.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
No conflict of interest declared.
Figures



Similar articles
-
OsWRKY21 and OsWRKY108 function redundantly to promote phosphate accumulation through maintaining the constitutive expression of OsPHT1;1 under phosphate-replete conditions.New Phytol. 2021 Feb;229(3):1598-1614. doi: 10.1111/nph.16931. Epub 2020 Oct 10. New Phytol. 2021. PMID: 32936937 Free PMC article.
-
OsPHT1;3 Mediates Uptake, Translocation, and Remobilization of Phosphate under Extremely Low Phosphate Regimes.Plant Physiol. 2019 Feb;179(2):656-670. doi: 10.1104/pp.18.01097. Epub 2018 Dec 19. Plant Physiol. 2019. PMID: 30567970 Free PMC article.
-
A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice.Plant Physiol. 2012 Aug;159(4):1571-81. doi: 10.1104/pp.112.196345. Epub 2012 May 30. Plant Physiol. 2012. PMID: 22649273 Free PMC article.
-
Environmental Control of Phosphorus Acquisition: A Piece of the Molecular Framework Underlying Nutritional Homeostasis.Plant Cell Physiol. 2021 Sep 24;62(4):573-581. doi: 10.1093/pcp/pcab010. Plant Cell Physiol. 2021. PMID: 33508134 Review.
-
Phosphate Uptake and Transport in Plants: An Elaborate Regulatory System.Plant Cell Physiol. 2021 Sep 24;62(4):564-572. doi: 10.1093/pcp/pcab011. Plant Cell Physiol. 2021. PMID: 33508131 Review.
Cited by
-
WRKY Transcription Factor Responses and Tolerance to Abiotic Stresses in Plants.Int J Mol Sci. 2024 Jun 21;25(13):6845. doi: 10.3390/ijms25136845. Int J Mol Sci. 2024. PMID: 38999954 Free PMC article. Review.
-
MicroRNAs as potent regulators in nitrogen and phosphorus signaling transduction and their applications.Stress Biol. 2024 Sep 12;4(1):38. doi: 10.1007/s44154-024-00181-x. Stress Biol. 2024. PMID: 39264517 Free PMC article. Review.
References
-
- Ai PH, Sun SB, Zhao JN, et al. . 2009. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. The Plant Journal 57, 798–809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

