BNT162b2 mRNA Vaccine-Induced Immune Response in Oral Fluids and Serum
- PMID: 36402577
- PMCID: PMC9671699
- DOI: 10.1016/j.identj.2022.09.005
BNT162b2 mRNA Vaccine-Induced Immune Response in Oral Fluids and Serum
Abstract
Objectives: The COVID-19 vaccine is currently being administered worldwide to address the ongoing pandemic. Although these vaccines have proven effective in preventing severe disease, the level of immunity required to prevent respiratory mucosal infection remains less well understood. Therefore, it is desirable to develop a noninvasive screening strategy such as oral fluid to monitor secreted antibodies longitudinally as potential surrogates of mucosal immunity.
Methods: We evaluated the anti-spike protein antibodies in gingival crevicular fluid (GCF) and saliva and compared them to immune responses in the blood of 50 healthy health care workers following 2 doses of intramuscular Pfizer/BioNTech-BNT162b2 vaccine.
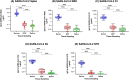
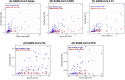
Results: The antibodies to SARS-CoV-2 spike and subdomain proteins (RBD, S1, S2, and NTD) were significantly higher in serum than oral fluids but showed a greater detection rate and higher median titres in GCF than saliva. For all tested SARS-CoV-2 antigens, IgG in GCF (as opposed to saliva) showed a more significant and stronger correlation with IgG in serum. Serum-neutralising antibodies (Nab) titres also displayed a significant and stronger correlation with anti-spike protein and their subdomains in GCF than saliva. Interestingly, the time post-second dose of vaccine and sex had a similar influence on IgG in serum and GCF. However, interferon (IFN)-γ-producing T-cell responses showed no association with SARS-Cov-2 IgG antibodies in serum, GCF, or saliva and neutralisation antibodies in serum. The correlation matrix of all measured parameters grouped serum and GCF IgG parameters separately from salivary IgG parameters indicating that GCF better represents the humoural response in serum than saliva.
Conclusions: Within limitations, we propose that GCF could be a less invasive alternative to serum and more appropriate than saliva to detect antibody responses by current COVID-19 vaccines if the GCF collection procedure could be standardised. Further research is needed to investigate the suitability of GCF for community immune surveillance for vaccines.
Keywords: COVID-19 vaccines; Gingival crevicular fluid; Immunity; Saliva.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest None disclosed.
Figures


Comment in
-
COVID-19 Vaccine-Induced Immune Response in Oral Fluids and Serum: Correspondence.Int Dent J. 2023 Apr;73(2):325. doi: 10.1016/j.identj.2022.12.002. Epub 2022 Dec 12. Int Dent J. 2023. PMID: 36610841 Free PMC article. No abstract available.
Similar articles
-
Antibody response to BNT162b2 mRNA vaccine in gingival crevicular fluid.J Periodontol. 2023 Jan;94(1):77-87. doi: 10.1002/JPER.22-0152. Epub 2022 Nov 11. J Periodontol. 2023. PMID: 35771077 Free PMC article.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Humoral immune response after different SARS-CoV-2 vaccination regimens.BMC Med. 2022 Jan 21;20(1):31. doi: 10.1186/s12916-021-02231-x. BMC Med. 2022. PMID: 35057798 Free PMC article.
-
Clinical Assessment of SARS-CoV-2 Antibodies in Oral Fluids Following Infection and Vaccination.Clin Chem. 2024 Apr 3;70(4):589-596. doi: 10.1093/clinchem/hvad169. Clin Chem. 2024. PMID: 38039096 Free PMC article. Review.
-
Impact of COVID-19 vaccination on saliva immune barriers: IgA, lysozyme, and lactoferrin.Arch Virol. 2023 Nov 16;168(12):293. doi: 10.1007/s00705-023-05914-3. Arch Virol. 2023. PMID: 37973637 Review.
Cited by
-
Long-term systemic and mucosal SARS-CoV-2 IgA response and its association with persistent smell and taste disorders.Front Immunol. 2023 Mar 8;14:1140714. doi: 10.3389/fimmu.2023.1140714. eCollection 2023. Front Immunol. 2023. PMID: 36969158 Free PMC article.
-
COVID-19 Vaccine-Induced Immune Response in Oral Fluids and Serum: Correspondence.Int Dent J. 2023 Apr;73(2):325. doi: 10.1016/j.identj.2022.12.002. Epub 2022 Dec 12. Int Dent J. 2023. PMID: 36610841 Free PMC article. No abstract available.
References
-
- Emergency Committee Regarding the Coronavirus Disease (COVID-19) Pandemic. April. 2005;19:2021.
-
- Nahass GR, Salomon-Shulman RE, Blacker G, et al. Intramuscular SARS-CoV-2 vaccines elicit varying degrees of plasma and salivary antibody responses as compared to natural infection. medRxiv. 2021 doi: 10.1101/2021.08.22.21262168. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

