Immunogenicity and Safety of SpikoGen, an Adjuvanted Recombinant SARS-CoV-2 Spike Protein, as a Heterologous Third Booster Dose in Kidney Transplant Patients: A Single-arm Clinical Trial
- PMID: 36402595
- PMCID: PMC9595368
- DOI: 10.1016/j.clinthera.2022.10.002
Immunogenicity and Safety of SpikoGen, an Adjuvanted Recombinant SARS-CoV-2 Spike Protein, as a Heterologous Third Booster Dose in Kidney Transplant Patients: A Single-arm Clinical Trial
Abstract
Purpose: Studies have found that immunocompromised patients have suboptimal responses to COVID-19 vaccines, leading to approval of a need for booster doses in this population. SpikoGen® is a subunit recombinant spike protein vaccine combined with Advax-CpG55.2™ adjuvant to protect against COVID-19. Previous clinical trials found this vaccine to be tolerable, immunogenic, and efficacious in reducing the risk of COVID-19, including severe disease. However, the effects of this vaccine have not been assessed in immunocompromised patients. This study sought to assess the immunogenicity and safety of the SpikoGen vaccine as a third booster dose in patients undergoing kidney transplant who were receiving immunosuppressive therapy and had received their primary vaccination based on an inactivated whole virus platform (Sinopharm).
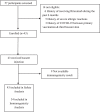
Methods: This single-arm trial was performed with 43 patients undergoing kidney transplant. The participants received a single booster dose of the SpikoGen vaccine 1 to 3 months after primary vaccination with 2 doses of the Sinopharm vaccine. Immunogenicity assessments were performed at baseline and 30 days after the booster dose. The primary outcomes were seroconversion rates of anti-S1 and surrogate virus neutralizing antibodies. Safety outcomes included the incidence of solicited and unsolicited adverse events in the 7 days and 1 month after the booster dose, respectively.
Findings: The SpikoGen vaccine induced positive humoral and cellular responses 30 days after the booster dose in those patients who were seropositive or seronegative after 2 primary doses of the Sinopharm vaccine. Thirty days after the SpikoGen vaccine booster, seroconversion rates were 35.29% (95% CI, 19.75%-53.51%) to anti-S1 and 29.41% (95% CI, 13.27%-46.57%) to surrogate neutralizing antibodies. The most common local and systemic reported solicited adverse events were injection site pain and fatigue, which were largely mild and transient. No serious adverse events were reported.
Implications: A single booster dose of SpikoGen vaccine given 1 to 3 months after primary vaccination with 2 doses of Sinopharm vaccine induced positive humoral and cellular immune responses in immunosuppressed patients undergoing renal transplant, thereby achieving spike antibody levels predictive of protection. This study was performed as a single-center study, and it will be important for future large multicenter studies to extend these results to other immunocompromised patient groups.
Keywords: Booster dose; COVID-19; Kidney transplant; SARS-CoV-2; SpikoGen.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interest SB, NA, HK, RSH, MB, and SK are members of the Orchid Pharmed medical department which is in collaboration with CinnaGen company with respect to conducting clinical trials. The remaining authors have no other relevant affiliations.
Figures
Similar articles
-
Clinical development of SpikoGen®, an Advax-CpG55.2 adjuvanted recombinant spike protein vaccine.Hum Vaccin Immunother. 2024 Dec 31;20(1):2363016. doi: 10.1080/21645515.2024.2363016. Epub 2024 Jun 5. Hum Vaccin Immunother. 2024. PMID: 38839044 Free PMC article. Review.
-
Immunogenicity and safety of SpikoGen®, an adjuvanted recombinant SARS-CoV-2 spike protein vaccine as a homologous and heterologous booster vaccination: A randomized placebo-controlled trial.Immunology. 2022 Nov;167(3):340-353. doi: 10.1111/imm.13540. Epub 2022 Jul 13. Immunology. 2022. PMID: 35758850 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of SpikoGen®, an Advax-CpG55.2-adjuvanted SARS-CoV-2 spike protein vaccine: a phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations.Clin Microbiol Infect. 2022 Sep;28(9):1263-1271. doi: 10.1016/j.cmi.2022.04.004. Epub 2022 Apr 15. Clin Microbiol Infect. 2022. PMID: 35436611 Free PMC article. Clinical Trial.
-
Immunogenicity and safety study of a single dose of SpikoGen® vaccine as a heterologous or homologous intramuscular booster following a primary course of mRNA, adenoviral vector or recombinant protein COVID-19 vaccine in ambulatory adults.Vaccine. 2025 Mar 7;49:126744. doi: 10.1016/j.vaccine.2025.126744. Epub 2025 Feb 5. Vaccine. 2025. PMID: 39914274 Clinical Trial.
-
The effectiveness of the first dose COVID-19 booster vs. full vaccination to prevent SARS-CoV-2 infection and severe COVID-19 clinical event: a meta-analysis and systematic review of longitudinal studies.Front Public Health. 2023 Jun 1;11:1165611. doi: 10.3389/fpubh.2023.1165611. eCollection 2023. Front Public Health. 2023. PMID: 37325336 Free PMC article.
Cited by
-
Clinical development of SpikoGen®, an Advax-CpG55.2 adjuvanted recombinant spike protein vaccine.Hum Vaccin Immunother. 2024 Dec 31;20(1):2363016. doi: 10.1080/21645515.2024.2363016. Epub 2024 Jun 5. Hum Vaccin Immunother. 2024. PMID: 38839044 Free PMC article. Review.
-
Exposure to unmethylated CpG oligonucleotides disrupts blood pressure circadian rhythms and placental clock gene network in pregnant rats.Am J Physiol Heart Circ Physiol. 2023 Aug 1;325(2):H323-H337. doi: 10.1152/ajpheart.00154.2023. Epub 2023 Jun 23. Am J Physiol Heart Circ Physiol. 2023. PMID: 37352412 Free PMC article.
-
Antibody Response Following the Intranasal Administration of SARS-CoV-2 Spike Protein-CpG Oligonucleotide Vaccine.Vaccines (Basel). 2023 Dec 20;12(1):5. doi: 10.3390/vaccines12010005. Vaccines (Basel). 2023. PMID: 38276664 Free PMC article.
-
The immunologic outcomes and adverse events of COVID-19 vaccine booster dose in immunosuppressed people: A systematic review.Prev Med Rep. 2024 May 31;44:102778. doi: 10.1016/j.pmedr.2024.102778. eCollection 2024 Aug. Prev Med Rep. 2024. PMID: 38979481 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous