Outbreak response strategies with type 2-containing oral poliovirus vaccines
- PMID: 36402659
- PMCID: PMC10284582
- DOI: 10.1016/j.vaccine.2022.10.060
Outbreak response strategies with type 2-containing oral poliovirus vaccines
Abstract
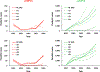
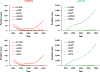
Despite exhaustive and fully-financed plans to manage the risks of globally coordinated cessation of oral poliovirus vaccine (OPV) containing type 2 (OPV2) prior to 2016, as of 2022, extensive, continued transmission of circulating vaccine-derived polioviruses (cVDPVs) type 2 (cVDPV2) remains. Notably, cumulative cases caused by cVDPV2 since 2016 now exceed 2,500. Earlier analyses explored the implications of using different vaccine formulations to respond to cVDPV2 outbreaks and demonstrated how different properties of novel OPV2 (nOPV2) might affect its performance compared to Sabin monovalent OPV2 (mOPV2). These prior analyses used fixed assumptions for how outbreak response would occur, but outbreak response implementation can change. We update an existing global poliovirus transmission model to explore different options for responding with different vaccines and assumptions about scope, delays, immunization intensity, target age groups, and number of rounds. Our findings suggest that in order to successfully stop all cVDPV2 transmission globally, countries and the Global Polio Eradication Initiative need to address the deficiencies in emergency outbreak response policy and implementation. The polio program must urgently act to substantially reduce response time, target larger populations - particularly in high transmission areas - and achieve high coverage with improved access to under-vaccinated subpopulations. Given the limited supplies of nOPV2 at the present, using mOPV2 intensively immediately, followed by nOPV2 intensively if needed and when sufficient quantities become available, substantially increases the probability of ending cVDPV2 transmission globally.
Keywords: Dynamic modeling; Eradication; OPV; Outbreak response; Polio.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
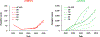
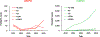
References
-
- Tebbens RJD, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal 2006;26(6):1471–505. - PubMed
-
- Thompson KM, Tebbens RJD. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal 2006;26(6):1423–40. - PubMed
-
- World Health Organization Global Polio Eradication Initiative. Polio eradication and endgame Strategic Plan (2013–2018) http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf; 2013. [accessed Jun 4, 2019].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

