Toxicity Index, patient-reported outcomes, and persistence of breast cancer chemotherapy-associated side effects in NRG Oncology/NSABP B-30
- PMID: 36402796
- PMCID: PMC9675799
- DOI: 10.1038/s41523-022-00489-9
Toxicity Index, patient-reported outcomes, and persistence of breast cancer chemotherapy-associated side effects in NRG Oncology/NSABP B-30
Abstract
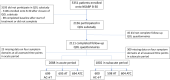
Adjuvant chemotherapy improves breast cancer survival but is associated with bothersome short- and long-term toxicity. Factors associated with toxicity, especially subacute toxicity up to 2 years following chemotherapy, have not been fully elucidated. The NRG Oncology/NSABP B-30 clinical trial compared 3 different doxorubicin-, cyclophosphamide-, and docetaxel-based chemotherapy regimens given over 3-6 months. Patients with hormone receptor-positive breast cancer received subsequent adjuvant endocrine therapy. From baseline through 24 months, 2156 patients completed questionnaires serially. We used multivariable probabilistic index models to identify factors associated with acute (>0-12 months) and subacute (>12-24 months) difficulties with pain, cognition, vasomotor symptoms, and vaginal symptoms. For all symptom domains, presence of symptoms prior to chemotherapy initiation were associated with symptoms in the subacute period (all p < 0.001). In addition, different combinations of patient factors and breast cancer treatments were associated with increased likelihood of pain, vasomotor, and vaginal symptoms in the subacute period. Consideration of pre-treatment symptoms and patient factors, as well as treatments for breast cancer, can facilitate identification of groups of patients that may experience symptoms following completion of chemotherapy. This information may be important for treatment-decision-making when alternative regimens are equivalent in benefit.
© 2022. The Author(s).
Conflict of interest statement
N.L.H. declares the following competing financial interest: payments to her institution for conduct of a pharmaceutical-sponsored clinical trial by Blue Note Therapeutics. P.A.G. declares the following competing financial interest: consulting income from Blue Note Therapeutics, GRAIL, and InformedDNA. The remaining authors declare no competing interests.
Figures


Similar articles
-
Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology).J Clin Oncol. 2017 Aug 10;35(23):2647-2655. doi: 10.1200/JCO.2016.71.4147. Epub 2017 Apr 11. J Clin Oncol. 2017. PMID: 28398846 Free PMC article. Clinical Trial.
-
Long-Term Follow-Up of Cardiac Function and Quality of Life for Patients in NSABP Protocol B-31/NRG Oncology: A Randomized Trial Comparing the Safety and Efficacy of Doxorubicin and Cyclophosphamide (AC) Followed by Paclitaxel With AC Followed by Paclitaxel and Trastuzumab in Patients With Node-Positive Breast Cancer With Tumors Overexpressing Human Epidermal Growth Factor Receptor 2.J Clin Oncol. 2017 Dec 10;35(35):3942-3948. doi: 10.1200/JCO.2017.74.1165. Epub 2017 Oct 26. J Clin Oncol. 2017. PMID: 29072977 Free PMC article. Clinical Trial.
-
Definitive results of a phase III adjuvant trial comparing six cycles of FEC-100 to four cycles of AC in women with operable node-negative breast cancer: the NSABP B-36 trial (NRG Oncology).Breast Cancer Res Treat. 2022 Jun;193(3):555-564. doi: 10.1007/s10549-021-06417-y. Epub 2022 Mar 1. Breast Cancer Res Treat. 2022. PMID: 35230585 Free PMC article.
-
Emerging role of taxanes in adjuvant and neoadjuvant therapy for breast cancer: the potential and the questions.Surg Clin North Am. 2003 Aug;83(4):943-71. doi: 10.1016/S0039-6109(03)00071-9. Surg Clin North Am. 2003. PMID: 12875604 Review.
-
Adjuvant chemotherapy in early breast cancer.Dan Med J. 2016 May;63(5):B5222. Dan Med J. 2016. PMID: 27127018 Review.
Cited by
-
Development of a Web-Based Interactive Tool for Visualizing Breast Cancer Clinical Trial Tolerability Data.JCO Clin Cancer Inform. 2024 Jul;8:e2400007. doi: 10.1200/CCI.24.00007. JCO Clin Cancer Inform. 2024. PMID: 39013121 Free PMC article.
-
Impact of social support on cognitive function in patients with breast cancer undergoing chemotherapy: The chain-mediating role of fatigue and depression.Asia Pac J Oncol Nurs. 2025 Jun 11;12:100743. doi: 10.1016/j.apjon.2025.100743. eCollection 2025 Dec. Asia Pac J Oncol Nurs. 2025. PMID: 40747250 Free PMC article.
-
Intensity-modulated proton radiotherapy spares musculoskeletal structures in regional nodal irradiation for breast cancer: a dosimetric comparison.Acta Oncol. 2024 Oct 1;63:755-762. doi: 10.2340/1651-226X.2024.40084. Acta Oncol. 2024. PMID: 39354810 Free PMC article.
-
Peri-Tumoural Lipid Composition and Hypoxia for Early Immune Response to Neoadjuvant Chemotherapy in Breast Cancer.Int J Mol Sci. 2024 Aug 28;25(17):9303. doi: 10.3390/ijms25179303. Int J Mol Sci. 2024. PMID: 39273252 Free PMC article.

