Host, technical, and environmental factors affecting QuantiFERON-TB Gold In-Tube performance in children below 5 years of age
- PMID: 36402803
- PMCID: PMC9675832
- DOI: 10.1038/s41598-022-24433-w
Host, technical, and environmental factors affecting QuantiFERON-TB Gold In-Tube performance in children below 5 years of age
Abstract
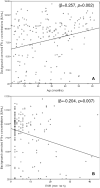
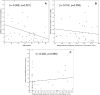
Interferon-gamma release assays performance can be impaired by host-related, technical and environmental factors, but data in young children are limited. We performed a cross-sectional study of children < 5 years-of-age at risk of tuberculosis (TB), using QuantiFERON-TB Gold In-Tube (QFT-GIT) assays. The impact of the following was evaluated: (i) host-related [age; hematological parameters; erythrocyte sedimentation rate (ESR); C-reactive protein (CRP); and tobacco smoke exposure (TSE) based on serum cotinine concentrations], (ii) technical (pre-analytical delay) and (iii) environmental factors (annual season; monthly temperatures). Of 204 children, 35 (17.2%) were diagnosed with latent TB infection or TB disease. QFT-GIT results were indeterminate in 14 (6.9%) patients. In multivariate analysis, younger age and higher ESR were associated with lower positive control responses (beta: 0.247, p = 0.002 and - 0.204, p = 0.007, respectively), and increasing age was associated with lower rates of indeterminate QFT-GIT results [OR (95% CI) 0.948 (0.903-0.996) per month, p = 0.035]. In children with positive QFT-GIT results, average monthly temperatures correlated with antigen responses (r = 0.453, p = 0.020); also, antigen responses were lower in winter than in other seasons (p = 0.027). Serum cotinine concentrations determined in a subgroup of patients (n = 41) indicated TSE in 36 (88%), positive control responses being lower in children with TSE (p = 0.034). In children < 5 years-of-age, young age, elevated ESR, temperature, annual season and TSE can affect the performance of QFT-GIT assays.
© 2022. The Author(s).
Conflict of interest statement
Dr. Marc TEBRUEGGE has received QuantiFERON assays at reduced pricing or free of charge for TB diagnostics projects from the manufacturer (Cellestis/Qiagen) in the past, and has received support for conference attendance from Cepheid. The manufacturers had no influence on the study design, data collection, analysis or interpretation, writing of the manuscript or decision to submit the data for publication. The rest of authors have no conflicts of interest to disclose.
Figures
References
-
- National Institute for Health and Clinical Excellence. Tuberculosis. NICE guideline. https://www.nice.org.uk/guidance/ng33 (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

