Folate receptor alpha in ovarian cancer tissue and patient serum is associated with disease burden and treatment outcomes
- PMID: 36402875
- PMCID: PMC9902484
- DOI: 10.1038/s41416-022-02031-x
Folate receptor alpha in ovarian cancer tissue and patient serum is associated with disease burden and treatment outcomes
Abstract
Background: Survival rates for ovarian cancer remain poor, and monitoring and prediction of therapeutic response may benefit from additional markers. Ovarian cancers frequently overexpress Folate Receptor alpha (FRα) and the soluble receptor (sFRα) is measurable in blood. Here we investigated sFRα as a potential biomarker.
Methods: We evaluated sFRα longitudinally, before and during neo-adjuvant, adjuvant and palliative therapies, and tumour FRα expression status by immunohistrochemistry. The impact of free FRα on the efficacy of anti-FRα treatments was evaluated by an antibody-dependent cellular cytotoxicity assay.
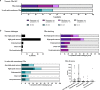
Results: Membrane and/or cytoplasmic FRα staining were observed in 52.7% tumours from 316 ovarian cancer patients with diverse histotypes. Circulating sFRα levels were significantly higher in patients, compared to healthy volunteers, specifically in patients sampled prior to neoadjuvant and palliative treatments. sFRα was associated with FRα cell membrane expression in the tumour. sFRα levels decreased alongside concurrent tumour burden in patients receiving standard therapies. High concentrations of sFRα partly reduced anti-FRα antibody tumour cell killing, an effect overcome by increased antibody doses.
Conclusions: sFRα may present a non-invasive marker for tumour FRα expression, with the potential for monitoring patient response to treatment. Larger, prospective studies should evaluate FRα for assessing disease burden and response to systemic treatments.
© 2022. The Author(s).
Conflict of interest statement
SNK and JS are founders and shareholders of Epsilogen Ltd. KFG is an employee of Epsilogen Ltd. HJB, MG, JL-A and LCGFP are employed through a fund provided by Epsilogen Ltd. SNK, JS, DHJ, HJB and KFG are inventors of patents on antibody technologies. All other authors have declared no conflict of interest.
Figures




References
-
- Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402. - PubMed
-
- Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505. - PubMed
-
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. - PubMed
-
- Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

