Study protocol for Vascular Access outcome measure for function: a vaLidation study In hemoDialysis (VALID) : A multi-center, multinational validation study to assess the accuracy and feasibility of measuring vascular access function in clinical practice
- PMID: 36402958
- PMCID: PMC9675211
- DOI: 10.1186/s12882-022-02987-1
Study protocol for Vascular Access outcome measure for function: a vaLidation study In hemoDialysis (VALID) : A multi-center, multinational validation study to assess the accuracy and feasibility of measuring vascular access function in clinical practice
Abstract
Background: A functioning vascular access (VA) is crucial to providing adequate hemodialysis (HD) and considered a critically important outcome by patients and healthcare professionals. A validated, patient-important outcome measure for VA function that can be easily measured in research and practice to harvest reliable and relevant evidence for informing patient-centered HD care is lacking. Vascular Access outcome measure for function: a vaLidation study In hemoDialysis (VALID) aims to assess the accuracy and feasibility of measuring a core outcome for VA function established by the international Standardized Outcomes in Nephrology (SONG) initiative.
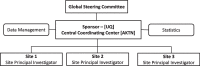
Methods: VALID is a prospective, multi-center, multinational validation study that will assess the accuracy and feasibility of measuring VA function, defined as the need for interventions to enable and maintain the use of a VA for HD. The primary objective is to determine whether VA function can be measured accurately by clinical staff as part of routine clinical practice (Assessor 1) compared to the reference standard of documented VA procedures collected by a VA expert (Assessor 2) during a 6-month follow-up period. Secondary outcomes include feasibility and acceptability of measuring VA function and the time to, rate of, and type of VA interventions. An estimated 612 participants will be recruited from approximately 10 dialysis units of different size, type (home-, in-center and satellite), governance (private versus public), and location (rural versus urban) across Australia, Canada, Europe, and Malaysia. Validity will be measured by the sensitivity and specificity of the data acquisition process. The sensitivity corresponds to the proportion of correctly identified interventions by Assessor 1, among the interventions identified by Assessor 2 (reference standard). The feasibility of measuring VA function will be assessed by the average data collection time, data completeness, feasibility questionnaires and semi-structured interviews on key feasibility aspects with the assessors.
Discussion: Accuracy, acceptability, and feasibility of measuring VA function as part of routine clinical practice are required to facilitate global implementation of this core outcome across all HD trials. Global use of a standardized, patient-centered outcome measure for VA function in HD research will enhance the consistency and relevance of trial evidence to guide patient-centered care.
Trial registration: Clinicaltrials.gov: NCT03969225. Registered on 31st May 2019.
Keywords: Arteriovenous fistula; Arteriovenous graft; Central venous catheter; Core outcome; Feasibility; Hemodialysis; Implementation; Patient engagement; Validation; Vascular access.
© 2022. Crown.
Conflict of interest statement
RRQ holds a patent for DMAR, a data collection platform for Q1 in dialysis and consultancy / research support Baxter Corporation. KM has consultancy fees from Otsuka Australia Pharmaceutical Pty Ltd., Support for professional meeting attendance from Amgen Australia and Roche Australia. DWJ has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care, consultancy fees from Astra Zeneca, Bayer, and AWAK, speaker’s honoraria from ONO and BI & Lilly, and travel sponsorships from Ono and Amgen. He is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant.
All other authors have no competing interests to declare.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

