Differential in vitro effects of targeted therapeutics in primary human liver cancer: importance for combined liver cancer
- PMID: 36402986
- PMCID: PMC9675209
- DOI: 10.1186/s12885-022-10247-6
Differential in vitro effects of targeted therapeutics in primary human liver cancer: importance for combined liver cancer
Abstract
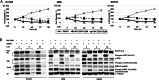
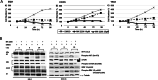
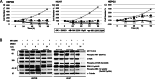
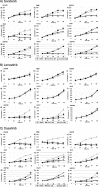
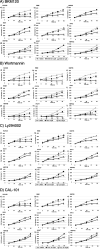
The incidence of primary liver tumors, hepatocellular carcinoma (HCC), intrahepatic cholangiocellular carcinoma (ICC), and combined HCC/ICC (cHCC/CC) is increasing. For ICC, targeted therapy exists only for a small subpopulation of patients, while for HCC, Sorafenib and Lenvatinib are in use. Diagnosis of cHCC/CC is a great challenge and its incidence is underestimated, bearing the risk of unintended non-treatment of ICC. Here, we investigated effects of targeted inhibitors on human ICC cell lines (HUH28, RBE, SSP25), in comparison to extrahepatic (E)CC lines (EGI1, CCC5, TFK1), and HCC/hepatoblastoma cell lines (HEP3B, HUH7, HEPG2). Cells were challenged with: AKT inhibitor MK-2206; multikinase inhibitors Sorafenib, Lenvatinib and Dasatinib; PI3-kinase inhibitors BKM-120, Wortmannin, LY294002, and CAL-101; and mTOR inhibitor Rapamycin. Dosage of the substances was based on the large number of published data of recent years. Proliferation was analyzed daily for four days. All cell lines were highly responsive to MK-2206. Thereby, MK-2206 reduced expression of phospho(p)-AKT in all ICC, ECC, and HCC lines, which mostly corresponded to reduction of p-mTOR, whereas p-ERK1/2 was upregulated in many cases. Lenvatinib showed inhibitory effects on the two HCC cell lines, but not on HEPG2, ICCs and ECCs. Sorafenib inhibited proliferation of all cells, except the ECC line CCC5. However, at reduced dosage, we observed increased cell numbers in some ICC experiments. Dasatinib was highly effective especially in ICC cell lines. Inhibitory effects were observed with all four PI3-kinase inhibitors. However, cell type-specific differences were also evident here. Rapamycin was most effective in the two HCC cell lines. Our studies show that the nine inhibitors differentially target ICC, ECC, and HCC/hepatoblastoma lines. Caution should be taken with Lenvatinib and Sorafenib administration in patients with cHCC/CC as the drugs may have no effects on, or might even stimulate, ICC.
Keywords: AKT pathway; Cholangiocellular carcinoma; Combined liver cancer; Hepatocellular carcinoma; Kinase inhibitors.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that no actual or potential conflict-of-interest in relation to this article exists.
Figures

References
-
- Adeva J, Sangro B, Salati M, Edeline J, La Casta A, Bittoni A, et al. Medical treatment for cholangiocarcinoma. Liver Int Off J Int Assoc Study Liver. 2019;39(Suppl 1):123–142. - PubMed
-
- Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int Off J Int Assoc Study Liver. 2019;39(Suppl 1):98–107. - PubMed
-
- American Cancer Society . Liver Cancer Survival Rates. 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

