Polymeric nanomedicines for the treatment of hepatic diseases
- PMID: 36402994
- PMCID: PMC9675156
- DOI: 10.1186/s12951-022-01708-y
Polymeric nanomedicines for the treatment of hepatic diseases
Abstract
The liver is an important organ in the human body and performs many functions, such as digestion, detoxification, metabolism, immune responses, and vitamin and mineral storage. Therefore, disorders of liver functions triggered by various hepatic diseases, including hepatitis B virus infection, nonalcoholic steatohepatitis, hepatic fibrosis, hepatocellular carcinoma, and transplant rejection, significantly threaten human health worldwide. Polymer-based nanomedicines, which can be easily engineered with ideal physicochemical characteristics and functions, have considerable merits, including contributions to improved therapeutic outcomes and reduced adverse effects of drugs, in the treatment of hepatic diseases compared to traditional therapeutic agents. This review describes liver anatomy and function, and liver targeting strategies, hepatic disease treatment applications and intrahepatic fates of polymeric nanomedicines. The challenges and outlooks of hepatic disease treatment with polymeric nanomedicines are also discussed.
Keywords: Hepatic fibrosis; Hepatitis B virus; Hepatocellular carcinoma; Host-versus-graft disease; Liver targeting; Nonalcoholic steatohepatitis; Polymeric nanomedicines.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




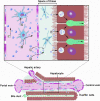
References
-
- Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71. - PubMed
-
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. - PubMed
-
- Geissler EK, Schlitt HJ. Immunosuppression for liver transplantation. Gut. 2009;58(3):452–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81901627/National Natural Science Foundation of China
- 52173145/National Natural Science Foundation of China
- U20A20360/National Natural Science Foundation of China
- 20210101063JC/Jilin Provincial Science and Technology Development Program
- 2022226/Youth Innovation Promotion Association of the Chinese Academy of Sciences
LinkOut - more resources
Full Text Sources
Medical

